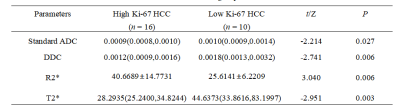
4249
The value of IVIM and enhanced T2star weighted angiography for preoperatively predicting Ki-67 status in hepatocellular carcinoma1Department of Radiology, The First Affiliated Hospital, Dalian Medical University, Dalian, Liaoning, China, China, 2Department of Pathology, The First Affiliated Hospital, Dalian Medical University, Dalian, Liaoning, China, China, 3Dalian Engineering Research Center for Artificial Intelligence in Medical Imaging, Dalian, Liaoning, China
Synopsis
In hepatocellular carcinoma (HCC) patients, high Ki-67 was closely related with tumor size, vein invasion, histological grade and the metastasis. This worked aimed at investigating the value of intravoxel incoherent motion (IVIM) diffusion-weighted magnetic resonance (MR) imaging and enhanced T2star weighted angiography(ESWAN) for predicting tumor Ki-67 status in HCC. The results showed that Standard ADC, DDC, R2*, T2* and combine had promising performances in predicting Ki-67 status. This research suggested that the IVIM and ESWAN held great potential in predicting the Ki-67 status of HCC, which is valuable for noninvasively, preoperatively and accurately predicting the prognostic factors during clinical practice.
Purpose
To investigate the value of intravoxel incoherent motion (IVIM) diffusion-weighted (DW) magnetic resonance (MR) imaging and enhanced T2star weighted angiography(ESWAN) for predicting tumor Ki-67 status in patients with hepatocellular carcinoma (HCC).Introduction
Hepatocellular carcinoma is the most common primary liver cancerwith increasing mortality[1,2]. Despite considerable advances in early diagnosis and surgical techniques, a great deal of HCC patients are still suffering from the poor prognosis[3]. Cell proliferation status plays a key role in tumor biology and affects prognosis and treatment efficacy [4]. Ki-67 protein is a marker that can suggest active cell proliferation [5]. In hepatocellular carcinoma (HCC) patients, high Ki-67 was closely related with tumor size, vein invasion, histological grade and the metastasis. Therefore, there should be a preoperative noninvasive way of predicting Ki-67 status to guide individualized HCC treatment and postoperative surveillance in clinical practices. Being able to simultaneously provide the quantification of perfusion and diffusion without the contrast agents, IVIM DW imaging has shown tremendous clinical potential for biomedical applications including cancer diagnosing, prognosis prediction, and therapeutic evaluation [6]. ESWAN can obtain the information of quantitative parameters such as phase value, T2*and R2* values to predict Ki-67 status in patients with HCC.Materials and Methods
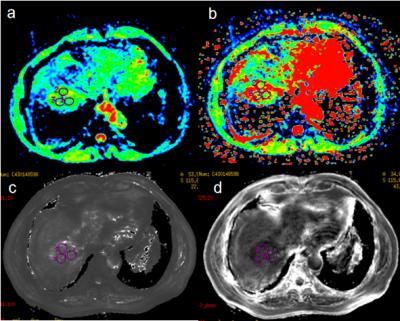
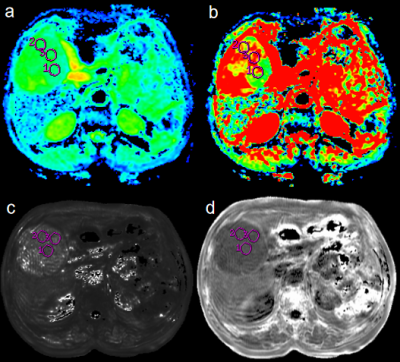
From June 2017 to April 2020, this study retrospectively collected 26 patients with surgically confirmed HCC who underwent abdominal MR examination before resection. The Ki-67 status was immunohistochemical determined and classified into low Ki-67 (labeling index < 15%) and high Ki-67 (labeling index ≥ 15%) groups. Aomong 26 patients, 16 patients with high Ki-67 status (12 males, age, 45-68 years), and 10 patients with low Ki-67 status (9 males, age, 31-80 years). All patients underwent abdominal 3.0T MR examinations (Signa HDxt, GE Medical Systems, USA), including T1WI, T2WI, contrast-enhanced MR, IVIM-DWI (b = 0, 20, 50, 100, 150, 200, 400, 800, 1200, 2000, 3000 s/mm2) and axial 3D ESWAN sequence. All the IVIM-DWI and ESWAN original data were processed using Functool software on GE AW 4.6 workstation. Quantitative IVIM-derived maps, including Standard apparent diffusion coefficient (ADC), Slow ADC Mono, Fast ADC Mono, Fraction of Fast ADC Mono, Slow ADC Bi, Fast ADC Bi, Fraction of Fast ADC Bi, DDC, and Alpha were automatically generated from IVIM DW imaging. ESWAN-derived maps included Magnitude, Phase, R2*, and T2*. The three regions of interest (ROIs) were placed on the maximum axial slice of the lesion (Figure 1 and 2) . The average value of three ROIs was used for further analysis. All statistic analyses were analyzed using SPSS 26.0 software. The above parameters between high Ki-67 and low Ki-67 groups were compared using Mann-Whitney U test. The logistic regression analysis was used to establish a predictive model of combining different parameters. Receiver operating characteristic (ROC) analysis was performed to evaluate predictive performance. The Delong’s test was used to compare the AUCs of different parameters.Results
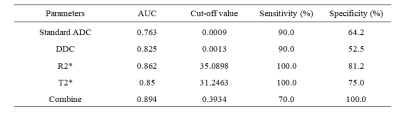
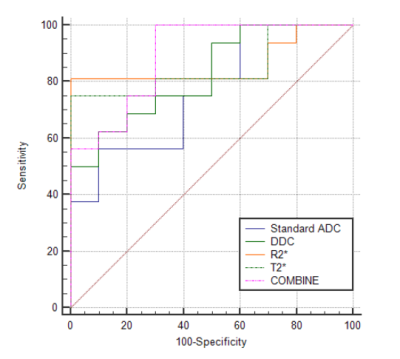
Comparisons of parameters between high Ki-67 HCC group and low Ki-67 HCC group were shown in Table1. The Standard ADC, DDC and T2* of high Ki-67 HCC group were lower than those of low Ki-67 HCC group, while the R2* of high Ki-67 HCC group were higher than those of low Ki-67 HCC group (P < 0.05). The area under the curve (AUC) of Standard ADC, DDC R2* and T2* for discriminating high Ki-67 HCC and low Ki-67 HCC were 0.763, 0.825, 0.862 and 0.850, respectively(Table2). When combined Standard ADC, DDC R2* and T2*, the diagnostic performance was improved (AUC, 0.894). ROC curve for discrimination of high Ki-67 HCC and low Ki-67 HCC is shown in Figure 3.Discussion and Conclusion
In recent years, IVIM and ESWAN has been widely used in the diagnosis, treatment efficacy evaluation and prognosis prediction of HCC[7-9]. The DDC of high Ki-67 HCC group was lower than those of low Ki-67 HCC group. DDC represents the average rate of diffusion[10]. Rapid proliferation of cancer cells, high cell density and tight arrangement in the high Ki-67 HCC group resulted in the reduction of extracellular space and limited diffusion of water molecules. The R2* was significantly higher in high Ki-67 HCC group than that in low Ki-67 HCC group. This may be due to more malformed neovascularization and higher metabolism in high Ki-67 HCC group, which leading to tumor being in a state of relative oxygen deficiency. In conclusion, the IVIM ESWAN might be potential in prediction of Ki-67 in patients with HCC, which suggested that the underlying quantitative image biomarkers is valuable for noninvasively, preoperatively and accurately predicting the prognostic factors during clinical practice.Acknowledgements
nothingReferences
[1]Luo Y, Ren F, Liu Y, Shi Z, Tan Z, Xiong H, Dang Y, Chen G. Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients: a meta-analysis. Int J Clin Exp Med. 2015 Jul 15;8(7):10235-47.
[2]Zhang Y, Kuang S, Shan Q, Rong D, Zhang Z, Yang H, Wu J, Chen J, He B, Deng Y, Roberts N, Shen J, Venkatesh SK, Wang J. Can IVIM help predict HCC recurrence after hepatectomy? Eur Radiol. 2019 Nov;29(11):5791-5803.
[3]Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006 Feb;243(2):229-35.
[4]Cao Y, Ke R, Wang S, et al. DNA topoisomerase IIαand Ki67 are prognostic factors in patients with hepatocellular carcinoma. Oncol Lett 2017;13:4109-16.
[5]Yang C, Zhang J, Ding M, Xu K, Li L, Mao L, Zheng J. Ki67 targeted strategies for cancer therapy. Clin Transl Oncol. 2018 May;20(5):570-575.
[6]Tang L, Zhou XJ. Diffusion MRI of cancer: from low to high b-values. J Magn Reson Imaging. 2019;49(1):23-40.
[7]Lee Y, Lee SS, Cheong H, Lee CK, Kim N, Son WC, Hong SM. Intravoxel incoherent motion MRI for monitoring the therapeutic response of hepatocellular carcinoma to sorafenib treatment in mouse xenograft tumor models. Acta Radiol. 2017 Sep;58(9):1045-1053.
[8]Peng J, Zheng J, Yang C, Wang R, Zhou Y, Tao YY, Gong XQ, Wang WC, Zhang XM, Yang L. Intravoxel incoherent motion diffusion-weighted imaging to differentiate hepatocellular carcinoma from intrahepatic cholangiocarcinoma. Sci Rep. 2020 May 7;10(1):7717.
[9]Tian SF, Liu AL, Liu JH, Li Y, Liu XD, Huang K, Song QW, Xu MZ, Guo WY. [Value of R2(*) in evaluating the biological behavior of primary hepatocellular carcinoma]. Zhonghua Yi Xue Za Zhi. 2016 Apr 19;96(15):1164-7.
[10]Guo R, Yang SH, Lu F, Han ZH, Yan X, Fu CX, Zhao ML, Lin J. Evaluation of intratumoral heterogeneity by using diffusion kurtosis imaging and stretched exponential diffusion-weighted imaging in an orthotopic hepatocellular carcinoma xenograft model. Quant Imaging Med Surg. 2019 Sep;9(9):1566-1578.
Figures