4246
Radiomics based on biparametric MRI for the detection of significant residual prostate cancer after androgen deprivation therapy1Fudan University Shanghai Cancer Center, Shanghai, China, 2GE Healthcare, Shanghai, China
Synopsis
The purpose of this study was to use the radiomics method to differentiate prostate cancer and benign peripheral zone (PZ) tissues and investigate its ability to detect significant residual prostate cancer based on whole-mount pathology. The mean ADC of significant residual lesions was significantly lower than that of CR/MRD, which was significantly lower than that of benign tissues. The efficiency of the radiomics models was similar to that of ADC in distinguishing CR/MRD from benign tissue and distinguishing residual from benign tissue. The radiomics models showed superior efficiency in differentiating significant residual from CR/MRD as compared to ADC.
Purpose
The global incidence of prostate cancer has been increasing in mostcountries.1 Androgen deprivation therapy (ADT) is a key primary treatment for advanced and metastatic prostate cancer and is an important neoadjuvant therapy before radiotherapy and surgery.2 Currently, the assessment of the ADT treatment effect is mainly based on the serum Prostate specific antigen (PSA) test, which cannot assess the changes in primary and metastatic lesions discriminatively.3 The biparametric (bp) MRI protocol based on diffusion weighted imaging (DWI) and T2 weighted imaging (T2WI) has shown great promise in the assessment of the effects of ADT in recent studies.4 However, most of these studies evaluated the effects of ADT by comparing the pre- and post-ADT MRI images without full pathological correlation. With the rise of radiomics, many studies have investigated the use of radiomics features for prostate cancer.5 The purpose of this study was to use the radiomics method to differentiate prostate cancer and benign peripheral zone (PZ) tissues and investigate its ability to detect significant residual prostate cancer based on whole-mount pathology.Methods
All the subjects gave written informed consent to participate the study, which was approved by the local ethical committee. Ninety-two prostate cancer patients underwent MRI after ADT and before prostatectomy (62 with significant residual disease and 30 with complete response or minimum residual disease [CR/MRD]). Totally 100 significant residual, 52 CR/MRD lesions, and 70 benign tissues were selected according to whole-mount pathology. First, 381 radiomics features were extracted for them on T2WI, DWI and apparent diffusion coefficient (ADC) maps. Optimal feature subsets were selected using support vector machine with recursive feature elimination algorithm (SVM-RFE). Then, ADC values of significant residual, CR/MRD lesions and benign tissues were compared by one-way analysis of variance. Logistic regression were used to construct models with remaining features for differentiation between cancer and normal tissues. Third, the efficiencies of ADC values and radiomics models for differentiating various tissues were assessed by Area Under Receiver Operating Characteristic Curve (AUC).Results
The mean ADC value(×10-3mm2 s-1) of significant residual lesions (1.10±0.02) was significantly lower than that of CR/MRD (1.17±0.02), which was significantly lower than that of benign tissues (1.30±0.02) (p<0.05)(Fig. 1-2). The efficiency of the radiomics models was similar to that of ADC in distinguishing CR/MRD from benign tissue (AUC: 0.77 vs0.79) and distinguishing residual from benign tissue (AUC: 0.83 vs 0.84). The radiomics models showed superior efficiency in differentiating significant residual from CR/MRD (AUC: 0.75 vs 0.56) as compared to ADC (Fig. 3).Discussion and Conclusion
Our results showed that radiomics could assess the appearance of prostate cancer after ADT and differentiate significant residual prostate cancer from benign and CR/MRD tissue. Radiomics approach could promote the detection of significant residual prostate cancer after ADT.Acknowledgements
No acknowledgement found.References
1. Teoh J, Hirai HW, Ho J, et al. Global incidence of prostate cancer in developing and developed countries with changing age structures. Plos One. 2019;14:e221775.
2. Komura K, Sweeney CJ, Inamoto T, et al. Current treatment strategies for advanced prostate cancer. Int J Urol. 2018;25:220-231.
3. Saini S. PSA and beyond: alternative prostate cancer biomarkers. Cell Oncol. 2016;39:97-106.
4. Hotker AM, Mazaheri Y, Zheng J, et al. ProstateCancer: assessing the effects of androgen-deprivation therapy using quantitative diffusion-weighted and dynamic contrast-enhanced MRI. Eur Radiol. 2015;25:2665-2672.
5. Alvarez-Jimenez C, Barrera C, Munera N, et al. Differentiating Cancerousand Non-cancerous Prostate Tissue Using Multi-scale Texture Analysis on MRI. IEEE Eng Med Biol Soc 2019:2695-2698.
Figures
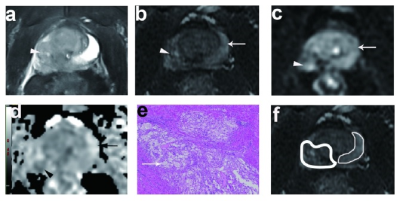
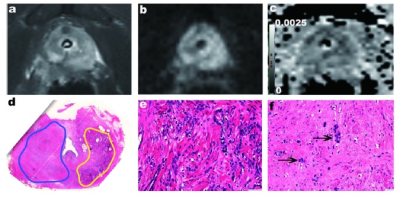
Fig, 2 A 68-year-old man with Gleason 5+4=9 prostate cancer underwent 3 months’ of ADT. (a)The post-ADT T2WI image. (b) On axial high-b value DWI, the left PZ showed high signal intensity, and the right PZ showed moderate signal intensity. (c) the ADC map showed low ADC values in the left PZ lesion and moderate values in the right PZ. (d) Photomicrograph of histopathology slide revealed residual on both sides of PZ. (e) The residual prostate cancer on the left PZ. (f) Right PZ characterized by reduction in gland size with decreased glandular density and increased peri-glandular density.
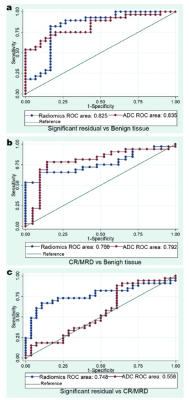
Fig. 3 (a, b) The AUCs of prediction models with the radiomics models for distinguishing CR/MRD from benign tissues and distinguishing significant residual from benign tissues were similar to those of ADC values. (c) The AUC of prediction model with the radiomics models for differentiating significant residual from CR/MRD was significantly higher than that of ADC values.