4227
The value of different diffusion models in evaluating liver regeneration after standard partial hepatectomy in rats1Radiology Department, Tianjin First Center Hospital, TIANJIN, China, 2Tianjin First Center Hospital, TIANJIN, China, 3Weihai Central Hospital, SHAN DONG, China, 4MR Collaboration, Siemens Healthcare Ltd., BEIJING, China, 5MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Different diffusion weighted imaging (DWI) models were used to evaluate microscopic changes of residual liver in rats after standard partial hepatectomy (70%PH). Multiple b-value DWI at multiple timepoints after 70%PH were acquired, and the data was analyzed using three diffusion models: conventional monoexponential DWI, intravoxel incoherent motion (IVIM), and diffusion kurtosis imaging (DKI) models. Compared with the monoexponential model of DWI, IVIM and DKI models can describe the changes of blood supply in the process of liver regeneration and provide added value in evaluating the microstructure of liver regeneration after standard partial hepatectomy.
Introduction
The liver has remarkable regenerative capabilities following acute or chronic injuries [1]. Partial hepatectomy (PH) is the preferred procedure for liver tumors and contributions from liver donors. Liver regeneration is important for restoring liver function after PH; failure of this process can result in postoperative hepatic dysfunction and liver failure [2, 3]. The complex processes and mechanisms of liver regeneration are unknown. Studies have shown that the residual liver will experience edema, sinusoidal dilatation, interstitial inflammatory cell infiltration, and hepatocyte proliferation after PH [4]. A conventional monoexponential diffusion weighted imaging (DWI) model can reflect the distribution and movement of water molecules in tissues. However, the biexponential model, also known as intravoxel incoherent motion (IVIM), can provide both perfusion and pure diffusion information by pseudo-diffusion coefficient (D*), perfusion fraction (f), and pure diffusion coefficients (D) [5, 6]. Diffusion kurtosis imaging (DKI) is a non-Gaussian model which is believed to better reflect the microstructure complexity in tissue using the mean diffusion coefficient (MD) and mean diffusion kurtosis (MK) [7]. The purpose of this study was to explore the value of different DWI models in evaluating liver regeneration in rats following standard partial hepatectomy.Materials and methods
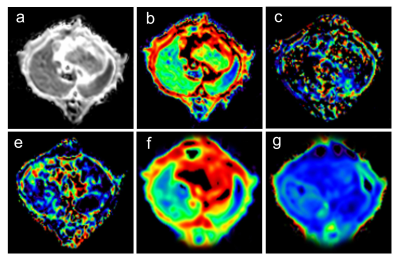
Fifty-six Sprague-Dawley rats were randomly divided into eight groups (n=7 per group). The 7 experimental groups were 1, 2, 3, 5, 7, 14, and 21 days after 70% PH, and 1 control group underwent a sham-operation. All rats were imaged using DWI with 13 b-values on a 3T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a dedicated 8-channel animal coil (Chenguang, Shanghai, China). Multiple b-value DWI was acquired using a single-shot echo-planar imaging (ss-EPI) sequence with the following parameters: TR/TE = 2300/74 ms; FOV = 120 × 98 mm2; slice thickness = 3 mm; matrix = 120 × 98; reconstructed voxel size = 0.5×0.5×3 mm3; acceleration factor = 4 (GRAPPA = 2; slice acceleration factor = 2); 13 b-values = 0, 10, 20, 30, 50, 100, 200, 300, 500, 800, 1000, 1500, and 2000 s/mm2 with three orthogonal directions for every b-value, and acquisition time =10 min 22 s. DWI data were used to compute the monoexponential, IVIM, and DKI models using a prototype postprocessing software (MR Body Diffusion Toolbox, Siemens). 2 b-values (0 and 1000 s/mm2) were used to calculate ADC by monoexponential DWI model; 10 b-values (0, 10, 20, 30, 50, 100, 200, 300, 500, and 800 s/mm2) were used to calculate D*, D, and f by the IVIM model; and 5 b-values (0, 500, 1000, 1500, and 2000 s/mm2) were used to calculate MD and MK by the DKI model. The differences of parameters derived from different DWI models were compared among different groups using a one-way ANOVA with Fisher’s least significant difference test. A P< 0.05 was considered statistically significant.Results
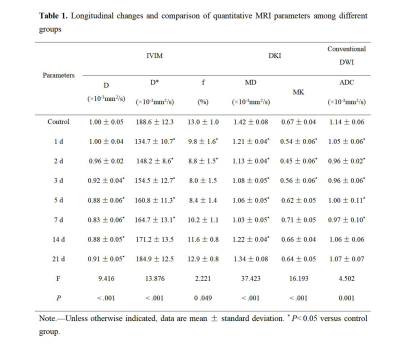
Table 1 displays parameter values and statistical testing for all groups. Compared with the control group, lower values across all parameters were observed in various groups after PH. D* values were significantly lower among 1 d, 2 d, 3 d, 5 d, and 7 d groups. Similarly, f values were significantly lower among 1 d and 2 d groups. In contrast, D values were significantly lower at later timepoint (3 d, 5 d, 7 d, 14 d, 21 d groups). MD values were significantly lower among 1 d, 2 d, 3 d, 5 d, 7 d and 14 d, while MK values were significantly lower among 1 d, 2 d, and 3 d groups. ADC values were significantly lower among 1 d, 2 d, 3 d, 5 d, and 7 d groups.Discussion
After PH, the content and microstructure of residual liver tissue changed significantly, and the diffusion of water molecules in the tissue was limited. D, MD, and ADC values, which reflect tissue diffusion, gradually decreased. At the same time, the residual liver tissue showed hepatocyte injury, hepatocyte edema, and hepatic sinus dilation, resulting in reduced heterogeneity of the tissue and water diffusion close to Gaussian distribution, and MK values decreased. On the other hand, hepatic cell edema, neutrophil infiltration and platelet aggregation in hepatic sinusoids occurred after PH, resulting in impaired hepatic microcirculation and significantly reduced tissue hemoperfusion. D* and f, which reflect tissue perfusion and perfusion fraction, decreased.Conclusions
In conclusion, IVIM and DKI can obtain multiple parameters which reflect tissue perfusion, tissue diffusion, tissue structure complexity, and deviation degree from Gaussian distribution in partial hepatectomy rats. In evaluating the quantitative accuracy of the rat partial liver resection model, it is superior to the traditional Gaussian model with mixed information.Acknowledgements
No acknowledgement found.References
1. Michalopoulos GK (2007) Liver regeneration. J Cell Physiol 213:286-300.
2. Yagi S, Hirata M, Miyachi Y, Uemoto S (2020) Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int J Mol Sci 21:8414.
3. van den Broek MA, Olde Damink SW, Dejong CH et al (2008) Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 28:767-780.
4. Harkness RD (1952) Changes in the liver of the rat after partial hepatectomy. J Physiol 117:267-277.
5. Le Bihan D (2008) Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology 249:748-752.
6. Le Bihan D (2017) What can we see with IVIM MRI. Neuroimage 187:56-67.
7. Jensen JH, Helpern JA, Ramani A, et al (2005) Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. MAGN RESON MED 53:1432-1440.