4223
High-Resolution Diffusion-Weighted Imaging of Hepatocellular Carcinoma (HCC)
Hui Zhang1, Chengyan Wang2, Peng Wu3, Huazheng Shi4, Weibo Chen3, and He Wang1
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Human Phenome Institute, Fudan University, Shanghai, China, 3Philips Healthcare, Shanghai, China, 4Shanghai Universal cloud imaging dignostic center, Shanghai, China
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Human Phenome Institute, Fudan University, Shanghai, China, 3Philips Healthcare, Shanghai, China, 4Shanghai Universal cloud imaging dignostic center, Shanghai, China
Synopsis
Diffusion-weighted imaging (DWI) is a widely used tool for diagnosing hepatocellular carcinoma (HCC). However, clinical routine liver DWI suffers from poor spatial resolution, severe geometry distortion, and image blurring since single-shot (SSH) echo-planar imaging (EPI) is used. Here we proposed an efficient solution for clinical high-resolution whole-liver DWI. The efficacy and generalization capability of the method were validated on healthy volunteers and HCC patients.
INTRODUCTION
Diffusion-weighted imaging (DWI) is a widely applied method for the diagnosis of hepatocellular carcinoma (HCC) due to its sensitivity to microscopic motion from either the water molecular Brownian motion or the blood microcirculation in biologic tissue1. However, clinical routine liver DWI suffers from poor spatial resolution, severe geometry distortion, and image blurring since single-shot (SSH) EPI is used, especially in locations with high field inhomogeneity2. The study of high-resolution DWI is rarely reported so far due to lots of challenges (such as motion control, signal-to-noise ratio (SNR) improvement). However, one comparative study was done on the liver with multi-shot echo-planar imaging (MSH-EPI) and SSH-EPI DWI, which chose conventional MUSE with only 2-shot and without any improvements on MSH-EPI clinical applications3. Hence, we proposed an efficient high-resolution DWI based on modified MUSE and validated the solution on 25 healthy volunteers and 5 HCC patients.METHODS
Sequence optimization: All MRI experiments were performed on a Philips 3.0 T clinical scanner (Philips Healthcare, Best, The Netherlands). The liver images were acquired from 25 healthy volunteers and 5 patients with lesions using a 32-channel SENSE Torso/Cardiac coil with a modified respiratory triggered multi-shot EPI DWI sequence, as shown in Figure 1. The optimizations included: a) SNR improved: To achieve minimum TE values, the total b values were assigned in multiple directions simultaneously to shorten the EPI acquisition window. The duration of diffusion gradients was compressed by using the maximum gradient strength as well as the slew rate multi-directions. b) Respiratory motion control: Free-breathing respiratory triggered scans with abdominal respiration and optimized trigger delay were recorded for image reconstruction. This helps to restrain the respiratory and cardiac motions with enough time to collect multi-shot EPI DWI data with multiple b-values. This helps to further correct phase variations among different shots. By this means, the accuracy of inter-shot phase calculation can be improved for further image analysis.Data acquisition: Four kinds of liver DWI protocols were acquired, covering the whole liver for 30 healthy volunteers and patients with different somatotypes. The detailed parameters are listed in Table 1. SSH-EPI and MSH-EPI DWI sequences (modified iEPI sequence without navigator echo) were scanned with 20 slices without slice gap. The in-plane resolution was 1.8×2 mm2 for MSH-EPI sequences and 3×3 mm2 for SSH-EPI sequences. Three b-values (for healthy volunteers: 0, 450 and 600 s/mm2; for patients: 0, 600 and 800 s/mm2) were chosen for MSH-EPI and SSH-EPI DWI. For MSH-EPI DWI, the full k-space was sampled by 4 independent shots. SSH-EPI DWI with the same echo train length (ETL) and TE were also acquired as a reference. All those images were acquired with half-scan factor of 0.712. Except considering the proper protocol for different subjects with different somatotypes, we also validate our method's robustness with different scan parameters (ETL and resolutions). As shown in Table 1, for thin subjects, SSH and MSH-EPI were acquired using scan#1 and scan#2, sharing the same ETL for comparison. For fatter subjects, data were acquired using scan#3 and scan#4. In addition, conventional T2-weighted images with a resolution of 1.6×1.6×6.0 mm3 using breath-hold were collected to provide anatomical information. Besides, the SNR of high-resolution images reconstructed by two methods was labeled in the bottom right corner, using the whole abdomen as the ROI for SNR calculation.
RESULTS
As shown in Figure 2, the results showed that our method was capable of providing high-resolution MSH-EPI DWI images with higher SNR (labeled in Figure 2 right corner) than the conventional regularized SENSE method and SSH-EPI DWI images. The image quality improvements for DWI were obvious with less signal loss (pointed by yellow arrows) and residual artifacts (labeled with red arrows), especially in those central regions of the images, where g-factor artifacts were severe. As depicted in Figure 3 for the results of HCC patients, the proposed method could provide the information about liver tissue with less signal loss in the central region of the liver (pointed by yellow arrows) and achieve more acceptable lesions delineation (indicated by red arrows) compared to the SSH-EPI and conventional MSH-EPI reconstruction algorithm. Besides, the experiments on volunteers and patients demonstrated the generalizability of our proposed method preliminarily.CONCLUSION
A whole-liver high-resolution solution, which helps improve image quality significantly, has been proposed. The results from healthy volunteers and patients with lesions demonstrated the feasibility and generalization performances of our method for high-resolution MSH-EPI DWI, which might be used for routine clinical applications as well as abdominal research.Funding: This work was supported by the National Natural Science Foundation of China (No. 81971583), National Key R&D Program of China (No. 2018YFC1312900), Shanghai Natural Science Foundation (No. 20ZR1406400), Shanghai Municipal Science and Technology Major Project (No.2017SHZDZX01, No.2018SHZDZX01) and ZJLab.
Acknowledgements
No acknowledgement found.References
1. Lebihan D, et al. Radiology 1988; 168:497. 2. Hui Z, et al. Neuroimage. 2021; 244: 118632. 3. Kim, Y. Y, et al. Eur J Radiol. 2020; 132:109292Figures

Figure.1 Pulse
sequence diagram and acquisition scheme for multi-shot interleaved EPI DWI.
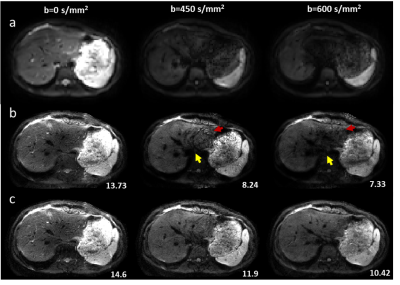
Figure.2 Representative
low-resolution from SSH-EPI (row a) and high-resolution DWI images from conventional
regularized SENSE reconstruction method (row b) and our method (row c) on
healthy volunteers. The SNR of reconstructed high-resolution DWI was labeled in
the bottom right corner.
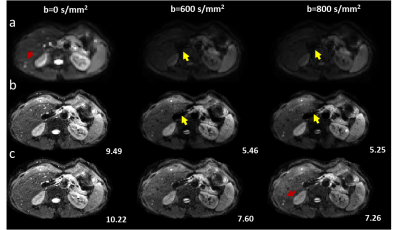
Figure.3 Representative
low-resolution from SSH-EPI (row a) and high-resolution DWI images from
conventional regularized SENSE reconstruction methods (row b) and our method
(row c) on patients. The SNR of reconstructed high-resolution DWI was labeled
in the bottom right corner.
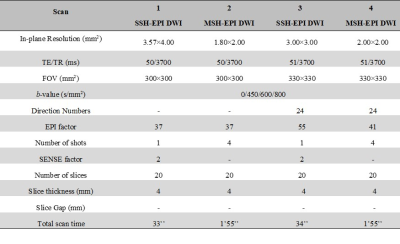
Table 1. Imaging parameters for this study
DOI: https://doi.org/10.58530/2022/4223