4216
Preoperative prediction of microvascular invasion in hepatocellular cancer using computation modeling of interstitial fluid pressure1Shanghai Institute of Medical Imaging, Shanghai, China, 2United Imaging Healthcare, Shanghai, China, 3Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China
Synopsis
Microvascular invasion (MVI) is a well-known major prognostic factor of hepatocellular carcinoma (HCC). Unlike macrovascular invasion, which can be easily identified via imaging before treatment, microvascular invasion (MVI) is visible only at microscopy. This study developed a new method for the noninvasive interstitial fluid pressure (IFP) measurement in HCC patients. Our results revealed that MVI status can be predicted by this IFP model preoperatively,
Introduction
Unlike macrovascular invasion, which can be easily identified via imaging before treatment [1], microvascular invasion (MVI) is visible only at microscopy, limiting its use as a prognostic factor for treatment allocation in real practice, regardless of its clinical importance. Recently, a non-invasive computational fluid modeling (CFM) to provide estimates of tumor interstitial fluid pressure (IFP) using the volume transfer constant (Ktrans) obtained from extended tofts model (ETM) emerged [2, 3]. The purpose of present study was to develop and validate a new method for the IFP measurement in hepatocellular carcinoma (HCC) patients. We also aimed to depict the microenvironment of the tumor of HCC and predict the MVI status preoperatively and noninvasively by this IFP model.Methods
Patients diagnosed between 2020 and 2021 that met the following inclusion criteria were included in this analysis: (1) histopathologic diagnosis of HCC; (2) single tumor with or without satellite nodules, which are lesions having a tumor diameter ≤2 cm and a distance from the main tumor ≤2 cm; (3) no preoperative cancer-related treatments, including transarterial chemoembolization and radiofrequency ablation; (4) no macrovascular invasion on MRI; and (5) an MR scan received within 1 month before curative hepatic resection or transplantation.MR imaging studies were performed using 3 T scanners (United Imaging Healthcare, Shanghai, China). DCE-MRI sequence utilizes a transverse T1-weighted spoiled GRE sequence to obtain acquisitions before, during and after the injection of a contrast agent bolus. A standard dose of 0.2 mL/kg of gadopentetate dimeglumine was injected intravenously at a rate of 2 mL/s. Immediately afterwards, a 20 mL saline flush was administered at the same injection rate. 60 time points were acquired with mean temporal resolution of 3.1 s and a total acquisition time of ~3 min. Patients were allowed to breathe freely.
The ETM was used to estimate the permeability parameters given by the Equation (1)
$$$Ctissue(t) = Ktrans∫0te-Ktrans/ve(t-τ)Cp(τ)dτ+vpCp(t)$$$ (1)
where Ktrans is the transfer constant, ve is the volume of extravascular extracellular space, vp is the fractional plasma volume, and Cp(t) is the time-course of plasma contrast agent concentration. The intravasation rate constant Kep can be calculated as Ktrans/ve.
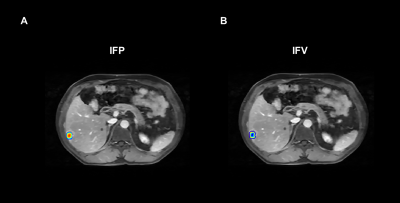
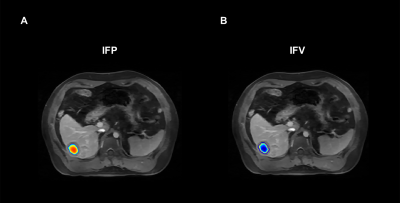
Volume of Interest (VOI) for Ktrans estimation were manually delineated on the tumor lesion present in all slices in the late phases of T1-weighted DCE-MRI images using 3D slicer (version 4.8.1; https://www.slicer.org), showing a clear tumor-to-normal tissue boundary.
As previously reported [4], the continuity partial differential equation (PDE) (Equation (2)) was implemented using the Matlab software
$$$ -KH∇2pi = Ktrans/<Ktrans>[LPS/V(pv-pi-σT(πv-πi))]-LpLSL/V(pi-pL)$$$ (2)
where pv and pi are pressures in vascular and interstitial space, respectively, πv and πi are the tumor osmotic pressure of the plasma and the interstitial fluid, Lp is the hydraulic conductivity of the vessel wall; S/V is the surface to volume ratio of the capillary wall, and σT is the osmotic reflection coefficient. Solving Equation (2) provides the basis for estimation of pressures in interstitial space and 3D parametric maps of IFP and interstitial fluid velocity (IFV).
Mann-Whitney U test was performed to compare the mean IFP and mean IFV between MVI-positive group and MVI-negative group. The receiver operating characteristic (ROC) analysis was performed for differentiation between MVI-positive group and MVI-negative group. The Youden index were exploited to determine the cutoff value, along with the area under the curve (AUC), sensitivity and specificity. P < 0.05 was considered statistically significant.
Results
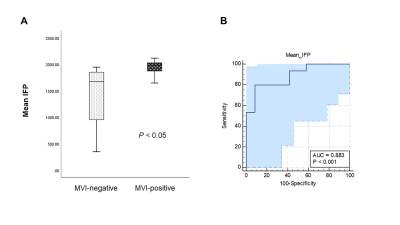
A cohort of 27 patients were finally included in this study (mean age 57.6 ± 10.9 years, 77.8% males). There were 15 patients in the MVI-positive group and 12 patients in the MVI-negative group. As shown in Figure 3A, Significant difference was found in IFP between the MVI-positive group and MVI-negative group (P = 0.001), while there was no statistically differences in IFV between the two groups (P = 0.626). For the ROC analysis (Figure 3B), IFP value proved to be the significant predictor with the best cut-off value 1.85 kPa (AUC = 0.883, 95% confidence interval 0.701 to 0.974; sensitivity = 80.0%, specificity = 91.7%).Discussion/Conclusion
MVI is a well-known major prognostic factor of HCC after surgical resection [5, 6], and liver transplantation [7, 8]. Accordingly, preoperative assessment of MVI is critical when developing treatment strategies for improved therapeutic outcomes among patients with HCC. This study proved that Mean tumor IFP estimated by CFM model was able to predict MVI in HCC. According to the previous literatures, elevated integral tumor pressure correlates to the presence of MVI [9]. One possible explanation could be the uncontrolled growth of cancer cells amplifies the metabolic activity of newly formed tissues which in turn increases the need for nutrients and oxygen. To provide nutrients and oxygen, enzymes was secreted to degrade the basement line of capillaries and promote the formation of new blood vessels and lymphatics. Then, once near a vessel, the proteases produced by the malignant cells degrade the endothelium of the vessel enabling the access of cells into the blood stream and the presence of MVI [10]. This procedure of angiogenesis leads to the increase of capillary permeability, blood flow resistance, and capillary pressure, which may result in the elevated IFP.Acknowledgements
No acknowledgement found.References
1. Liver, E.A.F.T.S.O.T., EASL clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology, 2018. 69(1): p. 182-236.
2. Swinburne, N., et al., Computational modeling of interstitial fluid pressure and velocity in non-small cell lung cancer brain metastases treated with stereotactic radiosurgery. Frontiers in neurology, 2020. 11: p. 402.
3. Paudyal, R., et al., Longitudinal Monitoring of Simulated Interstitial Fluid Pressure for Pancreatic Ductal Adenocarcinoma Patients Treated with Stereotactic Body Radiotherapy. Cancers, 2021. 13(17): p. 4319.
4. LoCastro, E., et al., Computational modeling of interstitial fluid pressure and velocity in head and neck cancer based on dynamic contrast-enhanced magnetic resonance imaging: feasibility analysis. Tomography, 2020. 6(2): p. 129-138.
5. Sumie, S., et al., Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Annals of surgical oncology, 2008. 15(5): p. 1375-1382.
6. Lim, K.-C., et al., Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Annals of surgery, 2011. 254(1): p. 108-113.
7. Shetty, K., et al., Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transplantation, 2004. 10(7): p. 911-918.
8. Iguchi, T., et al., New pathologic stratification of microvascular invasion in hepatocellular carcinoma: predicting prognosis after living-donor liver transplantation. Transplantation, 2015. 99(6): p. 1236-1242.
9. Sinkus, R., Elasticity imaging: from fibrosis and tumor pressure to mechanotransduction and visualizing primary neuronal activity.
10. Regge, D., G. Cappello, and A. Pisacane, Mechanism of Tumour Dissemination in Hepatobiliary and Pancreatic Tumours, in Hepatobiliary and Pancreatic Cancer. 2018, Springer. p. 1-12.
Figures