4215
Can Gd-EOB-DTPA enhanced MRI effectively in differentiate benign biliary obstruction from malignant obstruction?1Clinical Medical School of Yangzhou University, Northern Jiangsu People’s Hospital, Yangzhou, China, Yangzhou City, China, 2GE Healthcare, MR Research China, Beijing, P.R. China, Yangzhou City, China
Synopsis
This study aimed to investigate the feasibility of Gd-EOB-DTPA enhanced MRI in differentiating benign from malignant biliary obstruction at hepatobiliary phase (HBP) images. The liver enhancement ratio (LER) and the functional liver imaging (FLIS) score of benign and malignant biliary obstruction were significantly different. With these findings, Gd-EOB-DTPA enhanced MRI at HBP may be considered with an added clinical value in differentiating benign from malignant biliary obstruction.
Introduction
Biliary obstruction, divided into benign and malignant obstruction in the clinic, has complex etiology, including stones, inflammation, or neoplasm. However, as reported previously [1,2], accurate differentiation of benign and malignant biliary obstruction remains challenging, due to the lack of effective clinical or imaging features. Biliary system lesions, especially the obstruction of the low biliary duct, are easily misdiagnosed, severely affecting the decision-making of treatment plans and further prognosis of patients. Therefore, effective diagnosis including the accurate distinction between benign and malignant biliary obstruction is ideally required. Enhanced MRI with a liver-specific MR contrast agent of Gd-EOB-DTPA, has been reported to enhance bile duct significantly at hepatobiliary phase (HBP), allowing the good depiction of bile duct tree and thus providing direct diagnostic information for radiologists [3]. Of particular, Gd-EOB-DTPA enhanced MRI may hold more advantages to display anatomic features for biliary obstruction [4]. Therefore, this study mainly aimed to investigate the feasibility of Gd-EOB-DTPA enhanced MRI at HBP in differentiating benign from malignant biliary obstruction.Materials and Methods
SubjectsThe local ethical community approved the study and the consent forms were obtained from all patients. 143 patients with biliary obstruction were enrolled in the study, including 71 cases with benign obstruction determined by clinic diagnosis with follow-up imaging, and 72 cases with malignant obstruction confirmed by surgical pathology examination. All patients underwent Gd-EOB-DTPA enhanced MRI scan.
MRI experiment
MR experiments were performed on a 3.0-tesla scanner (MR750W, GE Healthcare, USA) using a 32-channel phased-array body coil. A liver acquisition with volume acceleration (LAVA) echo-based T1-weighted sequence was applied to the image from the top of the diaphragm to the lower margin of both kidneys prior to and post-contrast injection at the hepatobiliary phase (20 minutes after contrast injection). The corresponding scan parameters were applied including: TR=4.1ms, TE=1.9ms, slice thickness=5.4 mm, slice spacing=2.7mm, matrix size= 320×224, FOV= 40× 40cm2. All patients received a body weight-adapted dose (0.025 mL/kg body weight) of Gd-EOB-DTPA (Primovist; Bayer Schering Pharma AG, Berlin, Germany) intravenously administered as a bolus injection at a flow rate of 2.0 mL/s and flushed with 20ml 0.9% NaCl solution.
Data analysis
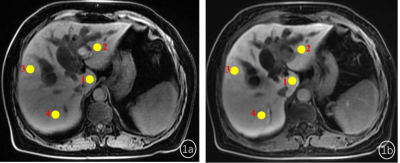
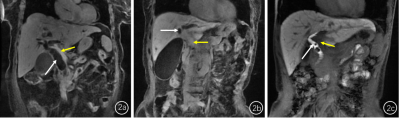
Two senior radiologists with 5-year and 10-year abdominal imaging experience manually delineated the region of interest (ROI, 50-100mm2)at the caudate lobe, left lobe, right anterior lobe and right posterior lobe hand on the same slice of T1 weighted images before and after contrast injection at hepatobiliary phase (Figure1). Mean signal intensity over liver four-segments based on T1 weighted images before contrast injection was recorded as SL0, and one of the liver four-segments based on post-T1w images at HBP was recorded as SL1. The liver enhancement ratio (LER) was calculated based on the equation of LER=(SL1-SL0)/SL0×100%. The functional liver imaging (FLIS) score (0-6 points) by visually evaluating liver parenchymal enhancement, biliary contrast excretion, and signal intensity ratio of portal vein relative to liver parenchyma [5,6] (Figure2).
Statistical analysis
Intraclass correlation coefficients (ICCs) analysis was performed to test the inter-rater agreement of LER and FLIS score evaluation between two radiologists. If an excellent agreement was obtained (ICC>0.8), mean levels of measurements were used for further analyses. Independent sample t-test was separately applied to compare the difference of liver parenchyma enhancement ratio and FLIS score between benign and malignant biliary obstruction. All statistical analyses were performed using SPSS software (SPSS version 22.0). P<0.05 was considered statistically significant.
Results
Excellent inter-rater agreement of evaluating LER and FLIS score between two radiologists was confirmed by high ICCs of 0.952 and 0.984, respectively. LER of patients with benign biliary obstruction was significantly higher than that of malignant biliary obstruction ((78 ± 43)% vs.(41 ± 27)%, p<0.001;Table 1). Moreover, similar pattern was also observed for FLIS scores between benign biliary obstruction and malignant biliary obstruction (4.17 ± 1.88 vs. 1.93 ± 1.70; p<0.001;Table 1).Discussion and Conclusion
Long-term biliary obstruction can cause cholestasis and hepatic pressure increase, leading to liver cell necrosis and apoptosis, and bile duct epithelial cell proliferation. Due to the decreased liver cells to absorb ethoxybenzyl (EOB) groups, the enhancement degree of liver parenchyma at HBP was decreased, the contrast agent metabolism through kidneys has a relative increase, and the portal vein signal intensity has a relative increase [7,8]. Malignant biliary obstruction is a long-term and chronic lesion. Its damaged liver cells are more than benign biliary obstruction. This study was implemented based on the above mechanism. The results showed that LER and FLIS scores of benign biliary obstruction patients were higher than those of malignant biliary obstruction. It is suggested that HBP imaging may provide added clinical value in distinguishing benign from malignant biliary obstruction. Other clinical examinations, such as laboratory tests, are often subjected to significant changes in a short period due to the influence of various transport mechanisms and drugs. In comparison, contrast-enhanced MRI at HBP with derived quantitative parameters of LER and FLIS score provided more direct information, helping for differentiating benign biliary obstruction from malignant obstruction. In conclusion, Gd-EOB-DTPA enhanced MRI at HBP may be considered effective in differentiating benign from malignant biliary obstruction.Acknowledgements
The authors thank the radiographers at our hospital for their cooperation.References
1.Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf). 2015; 3(1): 22-31.
2.Dorrell R, Pawa S, Zhou Y, et al. The Diagnostic Dilemma of Malignant Biliary Strictures. Diagnostics (Basel). 2020; 10(5): 337.
3.Lee NK, Kim S, Lee JW, et al. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009; 29(6): 1707-1724.
4. Kim DW, Kwon HJ, Kim KW, et al. Importance of Imaging Plane of Gadoxetic Acid--Enhanced Magnetic Resonance Cholangiography for Bile Duct Anatomy in Healthy Liver Donors. Transplant Proc. 2021; 53(1): 49-53.
5.Bastati N, Beer L, Mandorfer M, et al. Does the Functional Liver Imaging Score Derived from Gadoxetic Acid-enhanced MRI Predict Outcomes in Chronic Liver Disease? Radiology. 2020; 294(1): 98-107.
6.Lee HJ, Hong SB, Lee NK, et al. Validation of functional liver imaging scores (FLIS) derived from gadoxetic acid-enhanced MRI in patients with chronic liver disease and liver cirrhosis: the relationship between Child-Pugh score and FLIS. Eur Radiol. 2021; 31(11): 8606-8614.
7.Poetter-Lang S, Bastati N, Messner A, et al. Quantification of liver function using gadoxetic acid-enhanced MRI. Abdom Radiol (NY). 2020; 45(11): 3532-3544.
8.Talakic E, Steiner J, Kalmar P, et al. Gd-EOB-DTPA enhanced MRI of the liver: correlation of relative hepatic enhancement, relative renal enhancement, and liver to kidneys enhancement ratio with serum hepatic enzyme levels and eGFR. Eur J Radiol. 2014; 83(4): 607-611.
Figures