4213
Feasibility of Using Multi-frequency MR Elastography for the Assessment of Pancreatic Stiffness and Fluidity in Healthy Volunteers1The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
Synopsis
Tomoelastography, a newly emerging imaging modality, is a multi-frequency magnetic resonance elastography technique using noise-robust data post-processing. The aim of our study was to investigate the assessment of stiffness and fluidity of pancreas in healthy volunteers with tomoelastography. Tomoelastography derived pancreatic stiffness and fluidity were near-perfect reproducible in healthy volunteers. There was no stiff or fluidic difference among different groups of sex, age, BMI or pancreatic anatomical region. Tomoelastography can provide stable and promising stiffness and fluidity measurements throughout the pancreas. Our results provide data that will enable pancreatic studies of tomoelastography as a potential clinical tool in future.
Introduction
An accurate diagnosis of pancreatic cancers or chronic pancreatitis (CP) at an early stage remains challenging using current imaging approaches. New perspective for pancreatic disease imaging has been presented depends on the pancreatic mechanical properties. The fibroblast activation and intratumoral collagen deposition gradually develop dense fibrosis in pancreatic cancer [1]. CP is an inflammatory disease which induces progressive, irreversible fibrosis in pancreas [2]. These fibrosis leads to pancreatic stiffness increase which distinguishing from healthy pancreatic parenchyma. Moreover, as report goes, the fluidity increases are observed in pancreatic cancer and autoimmune pancreatitis as opposed to healthy people [3]. However, current imaging approaches are beneficial for the diagnosis of CP and pancreatic cancer, but the accuracy of early detection is still limited. Tomoelastography, a newly emerging imaging modality, is a multi-frequency magnetic resonance elastography (MRE) technique using noise-robust data post-processing [4]. Moreover, tomoelastography is potentially less affected by noise than single frequency MRE techniques [5]. It can quantify stiffness and fluidity of tissue from post-processed high-resolution and clear anatomy maps. The aim of our study was to investigate the assessment of stiffness and fluidity of pancreas in healthy volunteers with tomoelastography.Methods
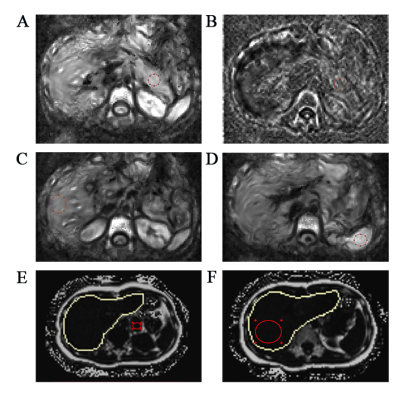
Forty seven healthy volunteers were enrolled in and underwent pancreatic MRI with tomoelastography. Two radiologists measured pancreatic stiffness and fluidity blindly and independently to determine the reproducibility. The stiffness and fluidity in pancreatic uncinated/head, body and tail were compared to determine anatomical difference. The mean pancreatic stiffness and fluidity were compared by gender, age and BMI respectively. Spearman correlation method was performed to determine correlation between other variables and pancreatic stiffness, fluidity respectively.Results
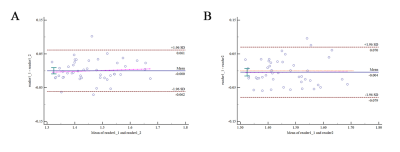
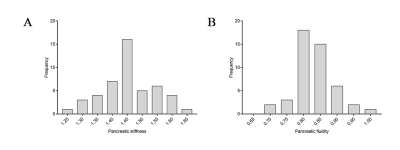
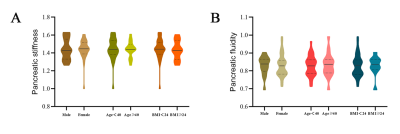
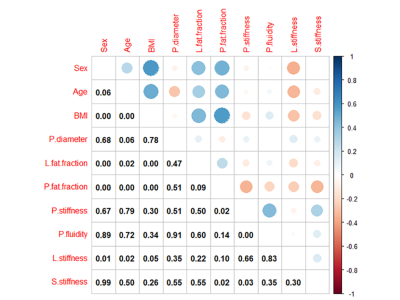
The Bland-Altman analysis demonstrated good agreement, the ICCs were 0.946 between two measurements of reader 1 and 0.922 between the measurements of reader 1 and reader 2 (both p < 0.001). There was no stiff (p = 0.477) or fluidic difference (p = 0.368) among pancreatic anatomical regions. The mean pancreatic stiffness was 1.45 ± 0.09 m/s and the pancreatic fluidity was 0.83 ± 0.06 rad. There was no stiff or fluidic difference among different groups of sex, age or BMI. The Spearman correlation coefficient (ρ = 0.375) was obtained when assessing the correlation between pancreatic stiffness and fluidity (p = 0.009).Discussion
Tomoelastography is potentially less affected by noise than other MRE techniques since tomoelastography uses multiple drivers and extracts shear wave speed through single-order derivative operators [5]. In standard direct inversion, this single-order finite-difference operator propagates less noise than a second-order operator [6]. In our study, the tomoelastography derived pancreatic stiffness was reproducible as the inter- and intra-observer agreements for pancreatic stiffness and fluidity measurements were near-perfect which in line with other researches [3, 5]. The pancreas can undergo age-related changes such as fibrosis, fat replacement, and lobular central atrophy [7, 8]. In theory, an aging pancreas should stiffening due to ongoing fibrosis and influx of lymphoplasmic cells [9]. On the other hand, parenchymal atrophy or fatty deposition may cause pancreas to soften [10]. The detailed mechanism behind the fibrogenesis and fatty deposition interactions on pancreatic stiffness remains further study. In addition, there was no regional variation in pancreatic stiffness values across the pancreatic sub-regions in healthy people with Stephan et al [5] which in line with ours with the same tomoelastography. This phenomenon further illustrates the stability and reproducible of measurement of pancreatic stiffness with tomoelastography. It is necessary and urgent to understand the role of pancreatic fluidity before apply to patients. However, at present, there are few studies that analyze the fluidity of tissues and organs in normal or diseased states. Fluidity conceptually means the change from a solid to a liquid state of a material. Recent researches showed that the fluidity of tissue is related to changes in intracellular structure and extracellular matrix rather than pure water content, suggesting the promising use in pathological changes [4, 11]. As Zhu et al [3] observed, fluidity increased in pancreatic cancer and autoimmune pancreatitis comparing with healthy people as the stiffness increased in these two diseases. These results provide impetus for exploring tomoelastography as a potential, stable and reliable tool to detect the fluidity of pancreatic diseases and more researches are needed to better understand the characteristic of pancreatic fluidity in future. There were several limitations to accurately interpret our results. First, the sample size in our study was relative small and larger BMI and older subjects with pancreatic atrophy and fatty infiltration will be need to involve in future studies. Second, these studies were conducted only in healthy volunteers, so further researches are needed to determine a reference range for specific diseases. Finally, the absence of pancreatic diseases in our volunteers were not confirmed through serological testing or other imaging examinations. Although the participants were thoroughly screened by asking about medical history and existing medical records, the possibility of unknown or unrecognized pancreatic disease still exists.Conclusion
Tomoelastography is a robust multi-frequency MR technique which can provide stable and promising stiffness and fluidity measurements throughout the pancreas. Our results afford data that will enable future studies of tomoelastography as a potential clinical tool.Acknowledgements
The authors sincerely thank Yangdi Wang and Xuefang Hu from The First Affiliated Hospital of Sun Yat-sen University for their technical support.References
1. Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29(3):179-87.
2. Sinha A, Singh VK, Cruise M, et al. Abdominal CT predictors of fibrosis in patients with chronic pancreatitis undergoing surgery. Eur Radiol. 2015;25(5):1339-46.
3. Zhu L, Guo J, Jin ZY, et al. Distinguishing pancreatic cancer and autoimmune pancreatitis with in vivo tomoelastography. Eur Radiol. 2021;31(5):3366-74.
4. Shahryari M, Tzschatzsch H, Guo J, et al. Tomoelastography Distinguishes Noninvasively between Benign and Malignant Liver Lesions. Cancer Res. 2019;79(22):5704-10.
5. Garcia SMR, Zhu L, Gultekin E, et al. Tomoelastography for Measurement of Tumor Volume Related to Tissue Stiffness in Pancreatic Ductal Adenocarcinomas. Investigative Radiology. 2020;55(12):769-74.
6. Tzschatzsch H, Guo J, Dittmann F, et al. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal. 2016;30:1-10.
7. Tariq H, Nayudu S, Akella S, et al. Non-Alcoholic Fatty Pancreatic Disease: A Review of Literature. Gastroenterology Res. 2016;9(6):87-91.
8. Matsuda Y. Age-related pathological changes in the pancreas. Front Biosci (Elite Ed). 2018;10:137-42.
9. Akkaya HE, Erden A, Kuru Oz D, et al. Magnetic resonance elastography: basic principles, technique, and clinical applications in the liver. Diagn Interv Radiol. 2018;24(6):328-35.
10. Xu Y, Cai X, Shi Y, et al. Normative Pancreatic Stiffness Levels and Related Influences Established by Magnetic Resonance Elastography in Volunteers. J Magn Reson Imaging. 2020;52(2):448-58.
11. Streitberger KJ, Lilaj L, Schrank F, et al. How tissue fluidity influences brain tumor progression. P Natl Acad Sci USA. 2020;117(1):128-34.
Figures