4207
Gadoxetic Acid–enhanced MRI for Predicting Postsurgical Recurrence in Hepatocellular Carcinoma:Compared with Four Clinical Staging Systems1Radiology, West China Hospital, Sichuan University, Chengdu, China
Synopsis
A total of 214 patients with pathologically confirmed Hepatocellular carcinoma (HCC) who underwent gadoxetic acid-enhanced magnetic resonance imaging (EOB-MRI) before curative resection between July 2015 and November 2020 were retrospectively included in this study. The preoperative model integrating EOB-MRI findings and serum AFP and AST levels achieved accurate recurrence prediction in HCC, with similar performance to that of the postoperative clinical-radiologic-pathologic model. Moreover, the preoperative model yielded superior predictive performance to four widely used clinical staging systems for HCC recurrence prediction. This model offered a potential noninvasive and reliable approach for individualized recurrence risk estimation before hepatectomy.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death, with growing incidence worldwide1, 2. Liver resection is the first-line curative option for HCC patients with well-preserved liver function3. Unfortunately, tumor recurrence occurs in ~50-70% cases within 5 years3, 4. Accurate risk estimation of recurrence is crucial to the individualized treatment, management and surveillance strategies. To date, several clinical staging systems, such as Barcelona Clinic Liver Cancer (BCLC) system, American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) system, Hong Kong Liver Cancer (HKLC) system, and Japan Integrated Staging (JIS) score, constitute the cornerstones in prognostic stratification and treatment allocation for HCC1, 2. Nevertheless, it could be challenging to predict HCC recurrence according to the above systems, because they are insufficient to profile the comprehensive landscape of tumor aggressiveness.Recently, encouraging evidences have been proposed on the potential value of gadoxetic acid-enhanced magnetic resonance imaging (EOB-MRI) for predicting recurrence in patients with HCC5-11. Despite the great potential of EOB-MRI, few studies have conducted a comprehensive assessment of tumor-related characteristics and proposed a noninvasive model for HCC recurrence with satisfactory predictive performance. Additionally, it is ambiguous whether the prognostic tools with the integration of novel imaging biomarkers would compete the conventional clinical staging systems for HCC recurrence prediction. To our knowledge, evidence remained scarce on comparing the prognostic value of preoperative EOB-MRI-based models with those of existing clinical staging systems.
Therefore, we aimed to investigate the effectiveness of integrating EOB-MRI, clinical, and pathologic parameters for postoperative HCC recurrence prediction in comparison with currently used staging systems.
METHODS
Consecutive patients with surgically confirmed HCC who underwent preoperative EOB-MRI between July 2015 and November 2020 were retrospectively included. All MR images were evaluated independently by two abdominal radiologists who were blinded to the outcomes. Two models, one incorporating only preoperative variables while the other incorporating all pre- and postoperative variables, were constructed via multivariable Cox regression analyses with five-fold cross-validation. Model performances were estimated by the Harrell's concordance index (C-index), time-dependent receiver operating characteristic curve analysis, and decision curve analysis. Comparisons were made between the preoperative model and postoperative model and four widely used clinical staging systems.RESULTS
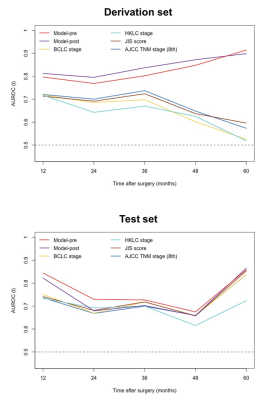
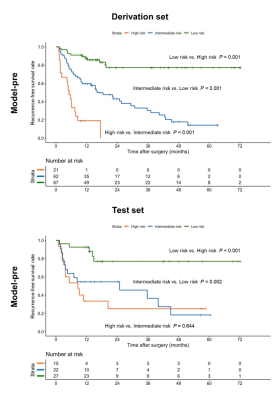
A total of 214 patients (derivation set: n = 150; test set: n = 64) were included, of whom 48.6% had tumor recurrence. Six preoperative variables, including tumor number, infiltrative appearance, corona enhancement, alpha-fetoprotein (AFP) level > 400 ng/mL, aspartate transaminase (AST) level > 40 IU/L, and male gender were independently associated with recurrence. After adding postoperative features, microvascular invasion (MVI) and poor tumor differentiation were additional significant variables in lieu of corona enhancement and AFP level. Using the above variables, the preoperative recurrence risk model achieved a C-index of 0.737 (95% confidence interval [CI]: 0.662, 0.812) on the test set, which was comparable with that of the postoperative model (0.727 [95%CI: 0.644, 0.809]; P = 0.733). Compared with four existing systems, the preoperative model yielded higher predictive accuracy and superior net benefit on both study sets. Additionally, patients with low, intermediate, and high risk for postsurgical recurrence were stratified according to the preoperative model. Furthermore, the frequencies of MVI were significantly distinct among low-, intermediate-, and high-risk groups, with 32.8% (22/67), 41.9% (26/62) and 90.5% (19/21) for the derivation set (P < 0.001) and 25.9% (7/27), 45.5% (10/22) and 86.7% (13/15) for the test set (P = 0.001), respectively.DISCUSSION
In the present study, the preoperative model incorporating EOB-MRI features and AFP and AST levels exhibited comparably satisfactory prognostic performance with that of the postoperative model, offering a potential noninvasive and reliable approach for preoperative individualized recurrence risk estimation. Moreover, the preoperative model yielded superior predictive performance to currently adopted clinical staging systems for HCC recurrence prediction. This model can be used to optimize surgical candidate selection, refine treatment protocols, individualize HCC management and tailor surveillance schedules, based on its recurrence risk stratification. This was to our knowledge the first study to directly compare the preoperative EOB-MRI-based model with four conventional staging systems for postoperative HCC recurrence prediction. Despite the merely slight advantages, novel clinical-radiologic biomarkers showed promise to improve the risk estimation of HCC recurrence to compensate for traditional staging systems. Further investigation is required to clarify the incremental value of novel imaging biomarkers to existing systems. However, the incorporation of semantic features solely is probably insufficient to optimize the prognostic scoring. More robust imaging biomarkers, such as semi-quantitative and quantitative parameters, need to be explored. It is worthwhile to note that the frequency of MVI increased significantly from low-, intermediate- to high-risk groups based on the preoperative recurrence risk stratification. These results shed light on the potential histopathologic mechanisms underlying the preoperative model in this study, unveiling the radiologic-pathologic-linkages.CONCLUSION
Integrating preoperative EOB-MRI features and serum AFP and AST levels allowed accurate recurrence prediction in HCC, with similar performance to that of the postoperative model. Moreover, the preoperative model yielded slight advantages over existing systems for HCC recurrence prediction. Further studies are required to investigate the incremental value of quantitative imaging biomarkers to conventional HCC staging systems.Acknowledgements
The authors declare no conflicts of interest.References
1. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750.
3. Vilarinho S, Calvisi DF. New advances in precision medicine for hepatocellular carcinoma recurrence prediction and treatment. Hepatology. 2014;60(6):1812-1814.
4. Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141(3):330-339.
5. Choi JW, Lee JM, Kim SJ, et al. Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR Images and their value as an imaging biomarker. Radiology. 2013;267(3):776-786.
6. An C, Kim DW, Park YN, Chung YE, Rhee H, Kim MJ. Single Hepatocellular Carcinoma: Preoperative MR Imaging to Predict Early Recurrence after Curative Resection. Radiology. 2015;276(2):433-443.
7. Kim S, Shin J, Kim DY, Choi GH, Kim MJ, Choi JY. Radiomics on Gadoxetic Acid-Enhanced Magnetic Resonance Imaging for Prediction of Postoperative Early and Late Recurrence of Single Hepatocellular Carcinoma. Clin Cancer Res. 2019;25(13):3847-3855.
8. Cha DI, Jang KM, Kim SH, Kim YK, Kim H, Ahn SH. Preoperative Prediction for Early Recurrence Can Be as Accurate as Postoperative Assessment in Single Hepatocellular Carcinoma Patients. Korean J Radiol. 2020;21(4):402-412.
9. Wei H, Jiang H, Zheng T, et al. LI-RADS category 5 hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MRI for early recurrence risk stratification after curative resection. Eur Radiol. 2021;31(4):2289-2302.
10. Ariizumi S, Kitagawa K, Kotera Y, et al. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18(4):575-585.
11. Lee S, Kim SH, Hwang JA, Lee JE, Ha SY. Pre-operative ADC predicts early recurrence of HCC after curative resection. Eur Radiol. 2019;29(2):1003-1012.
Figures