4205
T2-weighted dynamic approach for rectal MR imaging1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2United Imaging Healthcare, Shanghai, China, 3Department of Radiology, the First Hospital of Jilin University, Changchun, China
Synopsis
T2-weighted MR imaging is the standardized protocol in clinical evaluation of rectal cancer and anatomic structures. Usually, spasmolytic agent injection is required before the examination to reduce the imaging artifacts caused by the rectum peristalsis. In this work, we propose a novel dynamic imaging approach that with a complementary k-space sampling strategy and deep learning-based image reconstruction method to illustrate the anatomical structures of the rectum without any agent injection. Experimental results demonstrate the superior performance of the proposed approach.
Introduction
MRI is the preferred imaging modality for diagnosis and staging of rectal cancer, and T2-weighted imaging is the most important sequence for evaluating rectal tumors1,2. Good-quality MR images are important for recognizing and characterizing the tumor and its adjacent structures, especially the degree of tumor infiltration into normal tissues. However, due to the peristalsis of the rectum, administering spasmolytic agents such as hyoscine butylbromide is often required before the examination to reduce artifacts caused by peristalsis, which discomfort the patients3-5. In this work, we propose a novel high-resolution dynamic T2-weighted rectal MR imaging approach to reduce the artifacts caused by rectum peristalsis without any spasmolytic agent injection and capture the changes of the rectum during MR scan. Experimental results show the superior performance of the proposed approach.Theory
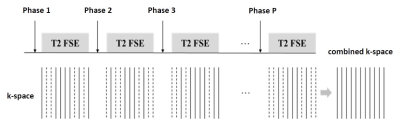
Data sampling:Due to the long TR and TE, the commonly used T2 FSE sequence in rectal cancer and anatomic structures evaluation can hardly achieve dynamic imaging, especially with high resolution. To facilitate the dynamic rectum imaging, we designed a new high-resolution 2D dynamic T2-weighted FSE sequence. With the creative complementary sampling strategy, each k-space phase encoding line would be collected in at least one temporal phase, which benefits image reconstruction. A full-filled k-space would be generated by combining the acquired k-space along the temporal direction. And the combined full-filled k-space can be used to estimate the coil sensitivity maps or reconstruction kernels relating to coil configuration. Fig 1 illustrates the proposed sampling strategy.
Image reconstruction:
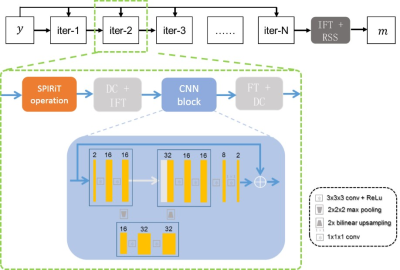
To improve the robustness of the reconstruction and reduce the computational complexity, we developed a new reconstruction method combining the SPIRiT model6 and deep learning. The objective function of the SPIRiT reconstruction can be formulated as $$\min_x{‖Dx-y‖_2^2+\lambda ‖Gx-x‖_2^2} (1)$$where $$$x\in C^N $$$ is the full k-space we wish to reconstruct from the undersampled k-space data $$$y\in C^M $$$, $$$ D:C^N→C^M$$$ is the sampling mask with $$$ N\geq M$$$, $$$ G $$$ is the SPIRiT operator. To improve the image quality, regularization is often used. Let $$$R(x) $$$ denotes the regularization, then the objective function becomes $$ \min_x{‖Dx-y‖_2^2+\lambda ‖Gx-x‖_2^2+R(x)} (2)$$With the POCS (Project OntoConvex Set) algorithm, the minimization problem (2) can be solved as follows: $$ \begin{cases}x^{n+\frac{1}{4}}=Gx^n \\x^{n+\frac{1}{2}}=(1-D)x^{n+\frac{1}{4}}+y \\ x^{n+\frac{3}{4}}=F(prox_{R,\tau} (F^H x^{n+\frac{1}{2}})) \\x^{n+1}=(1-D)x^{n+\frac{3}{4}}+y\end{cases} (3)$$where $$$ F$$$ denotes the Fourier transform. We replace the proximal operator $$$ prox_{R,\tau}$$$ with the learned operator $$$\Lambda $$$, the whole iteration can be rewritten as $$ \begin{cases}x^{n+\frac{1}{4}}=Gx^n \\x^{n+\frac{1}{2}}=(1-D)x^{n+\frac{1}{4}}+y \\ x^{n+\frac{3}{4}}=F(\Lambda (F^H x^{n+\frac{1}{2}})) \\x^{n+1}=(1-D)x^{n+\frac{3}{4}}+y\end{cases} (4)$$The illustrative diagram of the proposed method for dynamic rectum imaging is shown in Fig. 2. Five iterations were used and the output of each convolution network has two channels representing the real and imaginary parts of the dynamic MR data.
Method
The fully sampled dynamic rectum data were collected from 6 healthy volunteers on a 3T scanner (uMR790, United Imaging Healthcare, Shanghai, China) with IRB approval using an 18-channel receive coil and the standard 2D T2-weighted FSE sequence, TE/TR=106/3350ms, FOV=106$$$\times$$$380 mm, acquisition matrix = 288$$$\times$$$684, slice thickness=3mm. Each scan contains 15 slices in 1min 44 seconds, and we conducted 10 scans for each subject to simulate a 10-frame dynamic rectum raw data. As the motion of the rectum has no fixed pattern, 8 out of 10 frames were selected randomly to generate training samples, and each 10-frame raw dataset generated ten 8-frame datasets. The raw data of 4 volunteers were used to generate training samples, and the other two were used for testing. Overall 600 2D multi-coil rectum MR data were used for training.The prospective dynamic rectum data was acquired on the healthy volunteers with our designed 2D dynamic T2-weighted FSE sequence on the 3T scanner (uMR790, United Imaging Healthcare, Shanghai, China) with IRB approval. Relevant imaging parameters for 6-fold acceleration data were as follows: acquisition matrix = 256$$$\times$$$512, TE/TR=120/2600ms, slice thickness=3mm. Ten slices with 6 temporal frames were obtained, and the temporal resolution was 10s. And the protocol for 8-fold acceleration data was: FOV = 380$$$\times$$$320 mm, acquisition matrix = 420$$$\times$$$400, TE/TR=97.16/5738ms, slice thickness=5mm. Thirty slices with eight temporal frames were obtained.
Results
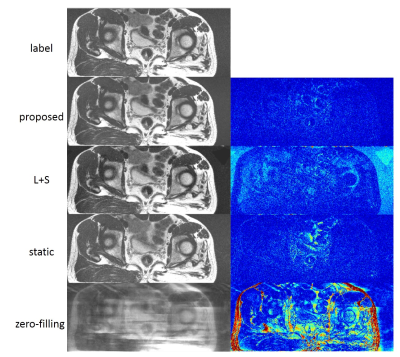
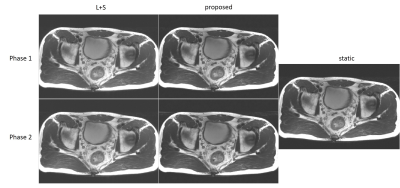
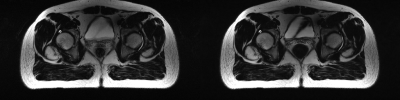
We compared the proposed dynamic imaging with the conventional static imaging, and compared the proposed DL-based reconstruction with low rank image reconstruction method L+S. The qualitative comparisons with acceleration factor of 8 are shown in Fig 3. The reconstructed images in the spatial domain, as well as the corresponding error maps were provided. The dynamic approach can achieve better performance than the conventional static imaging in illustration of the anatomic structures. And the proposed DL-based method can faithfully reconstruct the images with smaller errors and clearer anatomical details. Fig 4 and Fig 5 show the reconstructions of the prospective data. Compared to the standard static T2-weighted images, the reconstructed dynamic images can illustrate the movement of the rectum and provide a high-quality image at each phase in the temporal direction.Conclusion
In this work, we proposed a novel dynamic approach for T2-weighted rectal MR imaging. With the proposed complementary sampling strategy and the DL-based image reconstruction method, the anatomic structures and the peristalsis of the rectum can be illustrated very well, which may facilitate the evaluation and diagnosis of the rectal cancer in the future.Acknowledgements
This work was supported in part by the National Key R&D Program of China ( 2017YFC0108802 and 2017YFC0112903); China Postdoctoral Science Foundation under Grant (2020M682990,2021M693316); National Natural Science Foundation of China (62106252, 61771463, 81830056, U1805261, 81971611,61871373, 81729003, 81901736); Natural Science Foundation of GuangdongProvince (2018A0303130132); Innovation and Technology Commission of the government of Hong Kong SAR (MRP/001/18X); Strategic Priority Research Program of Chinese Academy of Sciences (XDB25000000).References
1. Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29(28):3753–3760.
2. Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28(4):1465–1475.
3. Jhaveri KS, Hosseini-Nik H. MRI of rectal cancer: an overview and update on recent advances. AJR Am J Roentgenol 2015;205(1):W42–W55.
4. Gollub MJ, Arya S, Beets-Tan RG, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY) 2018 May 21
5. Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics. 2019;39(2):367-387.
6. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med. 2010:64(2):457-71.
Figures