4201
Feasibility of an abdominal thin-slice breath-hold single-shot FSE sequence processed using a deep learning-based noise-reduction approach1Department of Radiology, International University of Health and Welfare Mita Hospital, Tokyo, Japan, 2Department of Radiology, International University of Health and Welfare Narita Hospital, Chiba, Japan, 3Department of Radiology, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan, 4Department of Radiology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan, 5International University of Health and Welfare, Tochigi, Japan
Synopsis
Single-shot fast spin echo (single-shot FSE) sequence is an accelerated T2-weighted imaging (T2WI) in pancreatic MRI. Fast advanced spin echo (FASE) is one of similar modalities. However, single-shot FSE suffers from image blurring and relatively low tissue contrast. We hypothesized that denoising approach with deep learning-based reconstruction (dDLR) would facilitate accelerated breath-hold thin-slice single-shot FSE MRI. We assessed the image quality of respiratory-triggered FSE T2WI (Resp-FSE) and breath-hold FASE with and without dDLR (BH-dDLR-FASE and BH-FASE, respectively) at 1.5 T. The image quality of BH-dDLR-FASE was superior to BH-FASE and Resp-FSE, and BH-dDLR-FASE had a shorter acquisition time than Resp-FSE.
Introduction
T2-weighted imaging (T2WI) without fat suppression is useful for evaluating the anatomy of the pancreas and surrounding structures1, and diagnosis of variable pancreatic diseases. But the long acquisition times and motion artifacts remain problematic2. Single-shot fast spin echo (single-shot FSE) imaging is an accelerated form of T2WI and useful for detection of cystic lesions3, and delineation of the main pancreatic duct (MPD)4. Fast advanced spin echo (FASE) is one of similar modalities. However, single-shot FSE is affected by image blurring because of T2 signal decay and relatively low tissue contrast1. Meanwhile, thin-slice T2WI is optimal for evaluating pancreatic disease, but higher spatial resolution MRI images have lower signal-to-noise ratios (SNRs). Recently, denoising approach with deep learning-based reconstruction (dDLR) effectively denoises MR images without compromising contrast5. We hypothesized that dDLR would facilitate accelerated breath-hold thin-slice single-shot FSE MRI, and reveal the pancreatic anatomy in detail. This study assessed the image quality of respiratory-triggered FSE T2WI (Resp-FSE) and breath-hold FASE with and without dDLR (BH-dDLR-FASE and BH-FASE, respectively) at 1.5 T without fat suppression.Methods
This study was approved by our Institutional Review Board.Patients and MR examinations
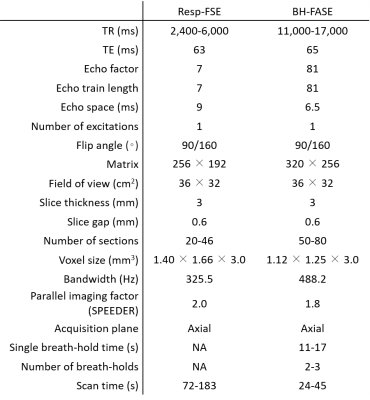
Forty-two patients (28 men, 14 women; mean age = 62.8 years; range: 34–89 years) who underwent abdominal MRI examinations because of suspected pancreaticobiliary disease were prospectively enrolled. MR examinations were performed using a 1.5-T scanner (Vantage Orian 1.5 T; Canon Medical Systems, Tochigi, Japan). We performed Resp-FSE-T2WI and BH-FASE sequences. The detailed imaging parameters are listed in Table 1.
dDLR technique and image processing
We used a commercial dDLR system, the Advanced Intelligent Clear IQ Engine (Canon Medical Systems), as reported previously5. The BH-FASE images were processed using the dDLR technique, which gave lower noise images (BH-dDLR-FASE).
Qualitative image analysis
Two board-certified radiologists (readers 1 and 2, with 18 and 27 years of imaging experience, respectively) visually evaluated the Resp-FSE, BH-FASE, and BH-dDLR-FASE images independently. They were blinded to clinical information and the image acquisition method, and all images were anonymized. Readers evaluated liver and pancreatic edge sharpness, the conspicuity of the main pancreatic duct (MPD) and adrenal glands, respiratory motion artifacts, coarseness, and overall image quality using 4-point scales from 1 (unacceptable) to 4 (excellent).
Quantitative image analysis
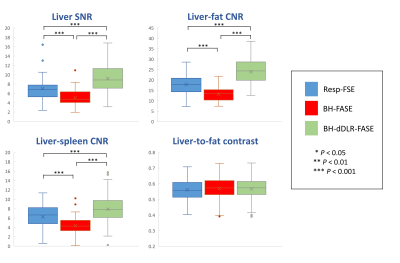
ROIs were drawn on the liver (in segment 6 or 7) and spleen parenchyma, and perihepatic fat surrounding the posterior margins of the liver, using ImageJ software (ver. 1.80; National Institutes of Health, Bethesda, MD, USA). The liver SNR, liver-fat contrast-to-noise ratio (CNR), liver-spleen CNR, and liver-to-fat contrast were calculated.
Statistical analysis
We used the Shapiro-Wilk test to explore the normality of the distribution of the data. We compared the Resp-FSE and BH-FASE acquisition times using the Wilcoxon signed-rank test. We used the Friedman test, followed by the post-hoc Wilcoxon signed-rank test with Bonferroni correction, for multiple comparisons of qualitative scores and nonparametric data (SNR data). We used repeated-measures analysis of variance (ANOVA) followed by post-hoc paired t-tests with the Bonferroni correction to analyze normally distributed CNR and contrast data. The Cohen quadratic-weighted kappa was used to assess inter-reader agreement. A p-value < 0.05 was considered statistically significant.
Results
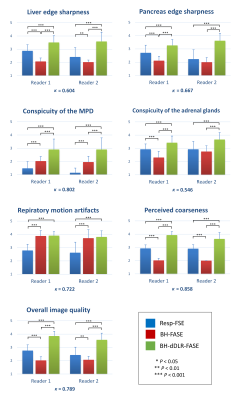
The acquisition time of BH-FASE was significantly shorter than that of Resp-FSE (30 ± 4 s vs 122 ± 25 s, p < 0.001).BH-dDLR-FASE was optimal in terms of liver and pancreas edge sharpness, adrenal gland conspicuity, coarseness, and overall image quality, followed by Resp-FSE and BH-FASE with significant differences among the three groups (Figure 3), with the exception of pancreas edge sharpness and adrenal gland conspicuity between Resp-FSE and BH-FASE for reader 2. The MPD conspicuity scores were highest for BH-dDLR-FASE, followed by BH-FASE and Resp-FSE. Respiratory motion artifacts did not differ between BH-dDLR-FASE and BH-FASE, but the Resp-FSE scores were significantly lower than those of BH-dDLR-FASE and BH-FASE. Weighted kappa statistics revealed moderate-to-excellent inter-reader agreement (kappa = 0.546–0.858).
The liver SNR, liver-fat CNR, and liver-spleen CNR were highest for BH-dDLR-FASE, followed by Resp-FSE and BH-FASE with significant differences (Figure 4). The liver-to-fat contrast did not differ among the three groups.
Discussion
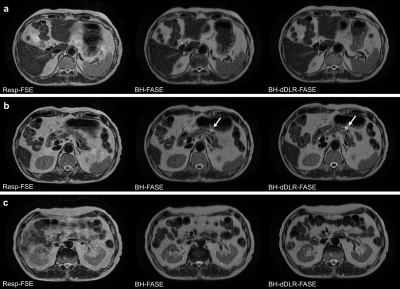
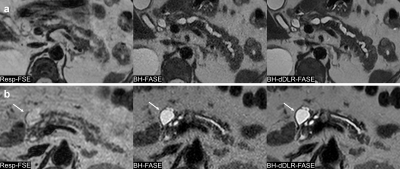
Qualitative and quantitative analyses showed that dDLR improved liver and pancreas sharpness, MPD and adrenal gland conspicuity, coarseness, overall image quality, and the liver SNR and CNR compared to those of original BH-FASE. All parameters were significantly better than those of Resp-FASE. Thus, dDLR aids abdominal thin-slice BH-FASE performed at 1.5 T, and thin-slice BH-dDLR-FASE is useful for evaluating the pancreas, which is a relatively thin organ surrounded by multiple anatomical structures. Given the high score for BH-dDLR-FASE, of almost 4 (excellent), it is clear that thin-slice BH-dDLR-FASE is clinically feasible. Moreover, it can be performed relatively quickly and motion artifacts are thus reduced.Although magnetic resonance cholangiopancreatography (MRCP) can reveal the entire MPD in detail, evaluation of extraductal structures is impossible. Thin-slice BH-dDLR-FASE allows precise evaluation of the anatomical relationships between peripancreatic organs and pancreatic cystic lesions, because cystic lesions present as clear high-intensity lesions on single-shot FSE (Figure 1, 2).
Conclusion
The image quality of abdominal thin-slice BH-FASE was significantly improved by denoising with DLR. Thin-slice BH-dDLR-FASE had a shorter acquisition time than Resp-FSE, and the image quality of the former was superior.Acknowledgements
None.References
1. Heyn C, Sue-Chue-Lam D, Jhaveri K, Haider MA. MRI of the pancreas: Problem solving tool. J Magn Reson Imaging. 2012;36:1037–51.
2. Klessen C, Asbach P, Kroencke TJ, Fischer T, Warmuth C, Stemmer A, et al. Magnetic resonance imaging of the upper abdomen using a free-breathing T2-weighted turbo spin echo sequence with navigator triggered prospective acquisition correction. J Magn Reson Imaging. 2005;21:576–82.
3. Kelekis NL, Semelka RC. MRI of pancreatic tumors. Eur Radiol. 1997;7:875–86.
4. Bogveradze N, Hasse F, Mayer P, Rupp C, Tjaden C, Klauss M, et al. Is MRCP necessary to diagnose pancreas divisum? BMC Med Imaging. 2019;19.
5. Kidoh M, Shinoda K, Kitajima M, Isogawa K, Nambu M, Uetani H, et al. Deep learning based noise reduction for brain MR imaging: tests on phantoms and healthy volunteers. Magn Reson Med Sci. 2020;19:195–206.
Figures