4187
Automatic segmentation and reconstruction of liver vascular structure on MRI with a deep learning model1Department of Radiology, The Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, 2Shukun (Beijing) Technology Co., Ltd, Beijing, China
Synopsis
The accurate segmentation of liver vascular structure is one of the key components of automated radiological diagnosis. Unfortunately, accurate vessel segmentation in clinical practice usually relies on the manual delineation by radiologists on each slice, which is extremely tedious and time-consuming. We developed and evaluated a new liver vessel segmentation and reconstruction workflow, which consist of a 3D Res-Net, a 2D Dense-Net and a 3D U-net model, allows for automated extraction of the portal vein, hepatic vein and inferior vena cava on 3D contrast enhanced portal venous phase MR images.
Introduction
Liver vascular structure analysis plays an important role for liver structural analysis, disease diagnosis and surgical planning [1]. For clinical use, the accurate segmentation of liver vessels is one of the key components of automated radiological diagnosis systems. However, influenced by the various vessel sizes, highly ramified branches, varying contrast and contain intensity distortions, accurate segmentation of liver vessels from abdominal MRI images is a challenging task [2]. In fact, accurate vessel segmentation in clinical practice usually relies on the manual delineation by radiologists on each slice, which is extremely tedious and time-consuming. Methods for liver vessel segmentation have been developed with Deep Learning (DL) networks based on different imaging techniques, but less for 3D MR images [3].The purpose of this work is to propose a fully automated liver vessel segmentation and reconstruction algorithm including portal vein, hepatic vein and inferior vena cava on 3D contrast enhanced MR images.Methods
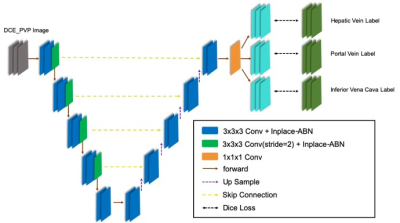
In this this study we respectively collected 258 patients with abdomen MR images from our hospital. And only portal venous phase (PVP) liver images acquired at around 60s post-injection were used. A total of 235 MR scans were included in the final cohort (105, 65, 65 for training, validation and testing respectively). The MRI images were reviewed by two radiologists and the target vessels on each slice of the patient were manually segmented on ITK-SNAP software. To ensure the consistency of the segmentation results, two radiologists went through a training session and segmented several cases together before the formal segmentation.A three-step workflow was used to automatically segment and reconstruct the liver vascular (Figure 1). Firstly, a classification 3D Res-Net(Res-Net18) model was used to identify the liver MR images . Secondly, based on the selected liver scans, a classification 2D Dense-Net model was used to identify the portal venous phase of the liver contrast-enhanced images [4]. Then a 3D U-net network was used to segment the selected PVP images (Figure 2). The 3D U-net architecture is consisted of the encoder and decoder part [5]. In the final layer, we used a 1 × 1 convolution network with a sigmoid activation function and a dice similarity coefficient loss function. The output of the segmentation decoder has three channels, and each channel corresponds to a type of blood vessel. In the convolutional blocks, In-Place Activated BatchNorm (INPLACE-ABN) [6] and Rectified linear unit (ReLU) are adopted to speed up the training and reduce the training memory. The edges of HV and PV are label smoothed so that the pixels at the edge of the blood vessel do not participate in the loss calculation.
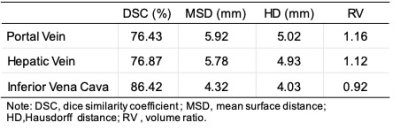
Dice loss function was used as the cost function. The segmentation accuracy of each vessel for the test datasets was evaluated using the dice similarity coefficient (DSC), mean surface distance (MSD), Hausdorff distance (HD) and volume ratio (RV). Simultaneously, the 3D structure of portal vein, hepatic vein and inferior vena cava can be reconstructed according to the segmentation results and voxel information.
Results
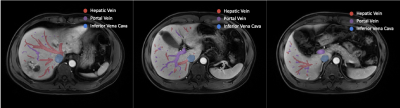
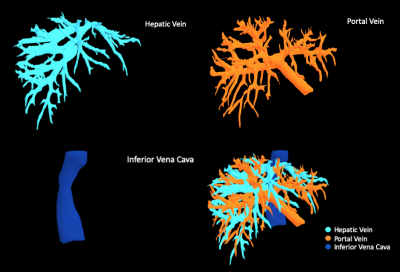
The dice loss and the segmentation accuracy of our model for the test datasets with each vessel are shown in Table 1. The DSC of HV, PV and IVC are 76.84%, 76.43% and 86.42%,respectively.The representative results of automatic segmentation are presented in Figure 3, different color indicates different vessels. And the 3D volume rending results are reconstructed and demonstrated in Figure 4.Discussion
Due to the complexity of 3D liver vessels, few machine learning based methods are used for liver-vessel segmentation. We have presented an deep learning based network for segmentation of vessels in the liver from MR image data sets and achieved high-accuracy segmentation results. The results show that the dice value of HV and PV are both around 0.76, while the dice value of IVC can reach 0.86. Because of the specific structure, the labeling errors of HV and PV may be greater than that of IVC, especially in the area of the end of the blood vessel. The different SNR of vessels may also cause the difference. Some large lesions (especially HCC and metastases) will squeeze and invade blood vessels, which will more affect HV and PV and leads to an unclear segmentation. As a future work, we will test the proposed method with more data set to improve.Conclusion
We developed and evaluated a new liver vessel segmentation and reconstruction workflow, which consist of a 3D Res-Net, a 2D Dense-Net and a 3D U-net model, allows for automated extraction of the portal vein, hepatic vein and inferior vena cava on 3D contrast enhanced portal venous phase MR images from a dynamic MRI acquisition with hepatospecific/extracellular contrast agents. Clinical implementation of the segmentation workflow is much more time efficient than manual liver vessel segmentation and provides consistent segment results, which may be helpful in disease diagnosis and liver resections.Acknowledgements
NoReferences
[1] D. Selle, B. Preim, A. Schenk, H.O. Peitgen. Analysis of vasculature for liver surgical planning. IEEE Trans Med Imag, 21 (2002), pp. 1344-1355.
[2] Goceri, E.; Shah, Z.K.; Gurcan, M.N. Vessel segmentation from abdominal magnetic resonance images: Adaptive and reconstructive approach. Int. J. Numer. Methods Biomed. Eng. 2017, 33, e2811.
[3] Ciecholewski, M.; Kassjański, M. Computational Methods for Liver Vessel Segmentation in Medical Imaging: A Review. Sensors 2021, 21, 2027.
[4] Huang G., Liu Z., Van Der Maaten L., and Weinberger K. Q., Densely connected convolutional networks, in Proc. IEEE Conf. Comput. Vis. Pattern Recognit. (CVPR), Jul. 2017, pp. 4700–4708.
[5] Çiçek, Ö., Abdulkadir, A., Lienkamp, S.S., Brox, T., Ronneberger, O. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. In: Ourselin, S., Joskowicz, L., Sabuncu, M.R., Unal, G., Wells, W. (eds.) MICCAI 2016. LNCS, vol. 9901, pp. 424–432. Springer, Cham.
[6] Bulo S.R., Porzi L., Kontschieder P. In-place activated batchnorm for memory-optimized training of DNNs; Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR); Salt Lake City, UT, USA. 18–23 June 2018.
Figures
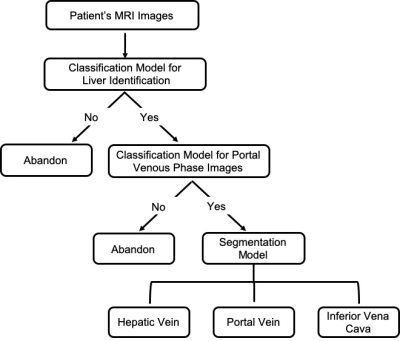
Figure 1. Overall framework of the proposed deep leaning model, including
image classification and liver vascular segmentation.