4185
Dual-domain self-supervised network for removing motion artifact related to Gadoxetic acid-enhanced MRI1State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Institute of Phys, Wuhan, China, 2Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 3Wuhan University of Technology School of Information Engineering, Wuhan, China, 4Philips Healthcare, Beijing, China, 5Wuhan University of Arts and Science, Wuhan, China, 6Henan University of Science and Technology, Luoyang, China
Synopsis
We proposed a dual-domain self-supervised motion artifacts disentanglement network (DSMAD-Net) for the liver's gadoxetic acid-enhanced arterial phase images. The motion correction is converted to the image-to-image translation problem by assuming that motion-free images and motion-corrupted images belong to different domains. Specifically, image-to-image translation within the same domain is designed to constrain auto-encoders to learn the feature representation by utilizing the input images as supervision information. Moreover, the cross-domain translation explores the cycle consistency in the absence of paired motion-free and motion-corrupted images. Experimental results demonstrate that our method remarkably removes artifacts in the gadoxetic acid-enhanced arterial phase images.
INTRODUCTION
Gadoxetic acid is a liver-specific MR imaging (MRI) contrast medium, which has been demonstrated to increase the detection of focal liver lesions1. However, there have been ample reports2,3 documenting “transient dyspnea” or “transient severe motion” frequently occurring after gadoxetic acid administration, which will result in degraded arterial phase images. Therefore, many prospective and retrospective strategies have been proposed to remove the motion artifacts. The prospective strategies, such as respiratory gating4, need additional hardware and long scan times. On the other hand, the traditional retrospective motion correction method rely on some a priori knowledge of the motion.In recent years, deep learning has been utilized to MR motion correction. Unlike traditional retrospective methods, these methods5,6,7 can learn the mapping from motion-corrupted data to motion-free data on the supervised manner. However, supervised approaches are not feasible for motion correction of gadoxetic acid-enhanced arterial phase images as the ground truth of non-rigid motion data is extremely challenging to obtain or simply not available. Therefore, several unsupervised methods8,9,10 have also been proposed to overcome the lack of paired data. However, blurring still exists as these unsupervised motion correction methods have not explored the properties of motion-affected images.
The goal of this work is to design a dual-domain self-supervised motion artifacts disentanglement network (DSMAD-Net) for the liver's gadoxetic acid-enhanced arterial phase images without any paired datasets. The results demonstrate that DSMAD-Net could effectively removes artifacts in the gadoxetic acid-enhanced arterial phase images.
METHODS
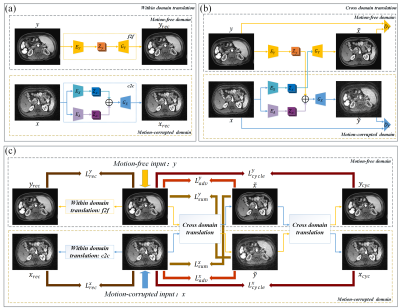
The dual-domain self-survised motion artifacts disentanglement network (DSMAD-Net) architecture is shown in Figure 1(c), it includes of 2 components:1) Within domain translation is shown in Figure 1(a), the main function of this component is to explore the input images themselves as supervision information to learn the content representation in the individual domain. We use the content encoder EY/EX to captures anatomical structures information, then the decoder GY/GX is applied to reconstruct the images yrec/xrec back. Especially, for the motion-corrupted images x, an addition artifact encoder EA is employed to disentangle the content component and artifact component.
2) Cross-domain translation (Figure 1(b)) is used to explore the cycle consistency in the absence of paired motion-free and motion-corrupted images. Different from the within domain translation, the decoder GY rather than GX is applied to generate the cross-domain translated motion-free image with the extracted content information zx. For the motion-free images y, we ensemble this anatomical content information zy and artifact features za extracted from motion-corrupted images x to construct the translated motion-corrupted image via the decoder GX.
Meanwhile, to clear the domain boundaries between motion-corrupted domain and motion-free domain, we employ the idea of generative adversarial networks. In our case, the decoders act as the generator network by translating an input image to a target-domain image. The two domain discriminators: motion-corrupted domain discriminator DX and motion-free domain discriminator DY are added to distinguish between real and fake images in respective domain.
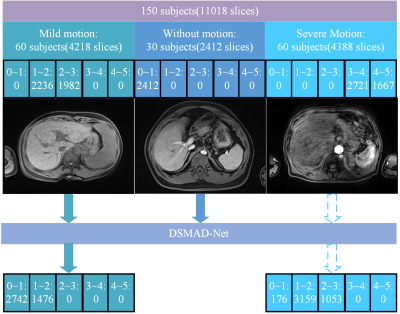
The model was externally validated in a dataset involving 55 gadoxetic acid-enhanced MRI examinations. To evaluate the artifacts of the arterial-phase images before and after motion correction, two study goals were set up:
1) Semi-quantitative assessments of artifact grades: using a five-point Likert scale based on the significance of the artifacts7,11,12.
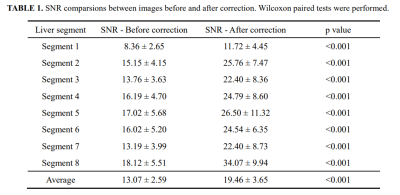
2) Objective assessments of the signal-to-noise ratios (SNRs) of the liver7,13,14: circular region of interests (ROIs) with the same size (𝜙2.0cm) were copied and pasted for the measurement of each liver segment (avoiding vessel and bile tract structures). In each liver segment, three ROIs at different locations were pasted in the axial images. Then, averaged signal intensities and standard deviation were recorded. Afterwards, SNR was calculated by dividing the averaged signal intensities by the standard deviation in each segment.
All these grading and measurements were performed by three independent radiologists with more than 10-year experience in abdominal radiology. The mean values were calculated and compared before and after motion correction by using paired Wilcoxon tests in SPSS software (version 26; IBM). A statistical significance was defined at a p value <0.001 level (two-tailed).
RESULTS
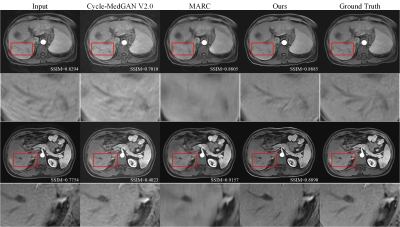
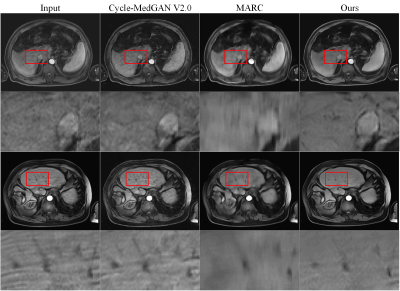
Figure 2 shows the comparison of the methods7,9 based on deep learning and this new DSMAD-Net method. We can notice that DSMAD-Net can reduce simulated motion artifacts in liver MR images and keep the anatomical details. Figure 3 shows the motion correction results with different correction methods for real in-vivo data.After testing this artifact correction in the arterial-phase images from an external dataset, it shows a significant decrease of the artifact grades from an average of 3.54±0.78 to 2.12±0.76 (p<0.001) (Figure 4). Besides, Table 1 shows the SNRs are significantly improved after correction compared with before correction at each liver segment of (p<0.001). The average of SNRs in all liver segments is 19.46±3.65 after correction, which is 13.07±2.59 before correction (p<0.001).
DISCUSSION & CONCLUSION
In this paper, we proposed a dual-domain self-survised motion artifacts disentanglement network (DSMAD-Net) for the liver's gadoxetic acid-enhanced arterial phase images without any paired datasets. The test in an external validation dataset demonstrates that our method remarkably removes artifacts in the gadoxetic acid-enhanced arterial phase images, possessing a potential for facilitating the radiologists to perform a more accurate diagnosis.Acknowledgements
We gratefully acknowledge the financial support by National Major Scientific Research Equipment Development Project of China (81627901), the National key of R&D Program of China (Grant 2018YFC0115000, 2016YFC1304702), National Natural Science Foundation of China (11575287, 11705274), and the Chinese Academy of Sciences (YZ201677).References
1. Wang Y C, Chou C T, Lin C P, et al. The value of Gd-EOB-DTPA-enhanced MR imaging in characterizing cirrhotic nodules with atypical enhancement on Gd-DTPA-enhanced MR images. PLoS One. 2017;12 (3): e0174594.2. Davenport M S, Caoili E M, Kaza R K, et al. Matched within-Patient Cohort Study of Transient Arterial Phase Respiratory Motion–related Artifact in MR Imaging of the Liver: Gadoxetate Disodium versus Gadobenate Dimeglumine. Radiology. 2014;272 (1): 123-131.
3. Luetkens J A, Kupczyk P A, Doerner J, et al. Respiratory motion artefacts in dynamic liver MRI: a comparison using gadoxetate disodium and gadobutrol. European Radiology. 2015;25 (11): 3207-3213.
4. Jiang S B. Technical aspects of image-guided respiration-gated radiation therapy. Medical Dosimetry. 2006;31 (2): 141-151.
5. Oksuz I, Clough J, Bustin A, et al. Cardiac MR Motion Artefact Correction from K-space Using Deep Learning-Based Reconstruction. Machine Learning for Medical Image Reconstruction. 2018: 21-29.
6. Armanious K, Jiang C, Fischer M, et al. MedGAN: Medical image translation using GANs. Computerized Medical Imaging and Graphics. 2020;79:101684.
7. Tamada D, Kromrey M L, Ichikawa S, et al. Motion Artifact Reduction Using a Convolutional Neural Network for Dynamic Contrast Enhanced MR Imaging of the Liver. Magn Reson Med Sci. 2020;19 (1): 64-76.
8. Armanious K, Jiang C M, Abdulatif S, et al. Unsupervised Medical Image Translation Using Cycle-MedGAN.27th European Signal Processing Conference (EUSIPCO). 2019.
9. Armanious K, Tanwar A, Abdulatif S, et al. Unsupervised adversarial correction of rigid MR motion artifacts. 2020 Ieee 17th International Symposium on Biomedical Imaging, New York: Ieee, 2020:1494-1498.
10. Ghodrati V, Bydder M, Ali F, et al. Retrospective respiratory motion correction in cardiac cine MRI reconstruction using adversarial autoencoder and unsupervised learning. NMR in Biomedicine. 2021;34 (2): 14.
11. Davenport, M.S., et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266(2): 452-461.
12. Pietryga, J.A., et al. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014;271(2): 426-434.
13. Havsteen, I., et al. Are Movement Artifacts in Magnetic Resonance Imaging a Real Problem?-A Narrative Review. Front Neurol. 2017;8:232.
14. Chen, F., et al. Variable-Density Single-Shot Fast Spin-Echo MRI with Deep Learning Reconstruction by Using Variational Networks. Radiology. 2018;289(2): 366-373.
Figures