4184
Motion-resolved and Free-breathing Liver MRF11. Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 23. Department of Health Technology & Informatics, The Hong Kong Polytechnic University, Hong Kong, Hong Kong, 3The Hong Kong Polytechnic University, Kowloon, Hong Kong
Synopsis
We developed a motion-resolved and free-breathing liver magnetic resonance fingerprinting (MRF) protocol. The deformation maps were obtained from the first singular image of MRF data. The reconstruction method enforced the consistency of the MRF data with the deformation maps by adding the deformation maps to the encoding matrix. MRF was tested on four healthy volunteers. We demonstrated a motion-resolved MRF with a nominal frame rate of 2.5 Hz for free-breathing liver imaging.
Introduction
Recently, rigid and non-rigid motion correction methods have been used in MRF reconstruction to reduce motion artifacts in brain, liver, and heart MRF experiments (1–4). The previous abdominal MRF studies were mostly breath-hold based (5,6). Our recent study showed the feasibility of free-breathing liver MRF in normal volunteers (7). However, no study performed free-breathing liver MRF in a 13-seconds/slice speed with motion-resolved reconstruction. In this study, we proposed to apply non-rigid image registration to complete the time series at each time frame for the motion-resolved MRF reconstruction.Methods
The local institutional research ethics committee approved the in vivo experiments. The MRF scan was performed on a GE 3T system (GE Healthcare). A 50-channel “AIR” coil was used for the signal reception. A FIDALL sequence was used for the MRF scan with parameters: a “half-sine”-style variable flip angle, 1000 dynamics, spiral out readout, 2D FISP acquisition, constant TE = 1.77 ms, repetition time (TR) varied from 13-16 ms, slice thickness = 5 mm, matrix size = 256 × 256, field of view 300 × 300 mm2, number of TRs = 1000, number of total spiral interleaves = 377, uniform rotation of spiral (360⁰/377), and scan time = 13 s/slice. In last 4D MRF scan, the parameters were slice thickness = 7 mm, slice spacing = 2 mm, number of slices = 20, and scan time = 4 min 18 sec. The following ranges were used for dictionary generation and dictionary matching: 10.2 < T1 < 4000 ms and 1.1 < T2 < 2000 ms.The sensitivity maps were compressed to 12 singular value components with a threshold at 20% of the largest singular value. The k-space data and dictionary were also compressed into five singular value components, using a threshold at 5% of the largest singular value measured on the dictionary elements. Finally, the reconstruction was performed using gradient descent in MATLAB (MathWorks, Natick). Gradient descent for reconstruction The cost function for MRF reconstruction is given as$$L(x^*) = ||WFSMUx^*-WUy^*||_2^2+R(x^*) $$
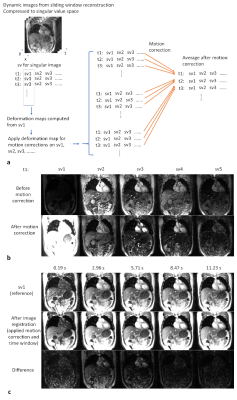
where x* “virtually complete” singular images, i.e., MRF time series at each time frame after compression, y* singular k-space data after compression, R regularization, S the coil sensitivity profiles, F non-uniform Fourier transform (NUFFT), W the undersampling mask with sliding window operator, U first k singular vectors of dictionary for data compression (we set k =5 in this study), and M the transpose of motion correction operator as shown in Figure 1. Minimizing the L(x*) allows the reconstruction of the MRF image series. Two L1-wavelet constraints apply to spatial and temporal features of singular images. This regularization is similar to the one used in CINE MRF reconstruction (4).
Results
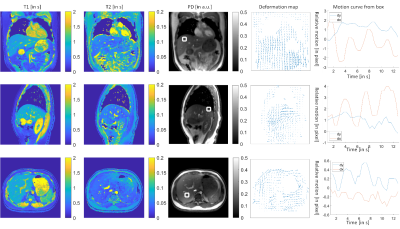
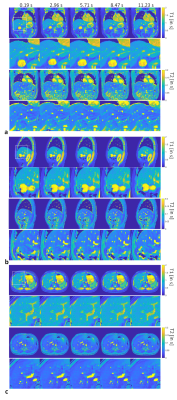
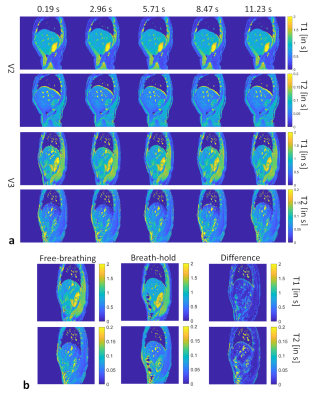
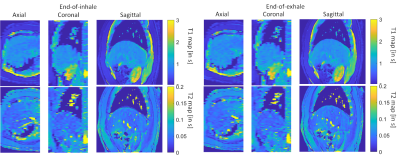
The motion correction method is illustrated in Figure 1. The MRF raw time series were segmented into 33 time frames and compressed into singular values. Figure 1b shows the results from the proposed motion correction method. Figure 1c demonstrates the accuracy of the motion correction on the dynamic MRF singular images. The NRMSE was 0.19 for measuring the difference between the reference singular image and singular image after motion correction. In Figure 2, the proposed method was robust enough to extract the pixel-wise movement patterns for all liver MRF scans with 0.39 s/frame resolution. Figures 3 shows images for five time frames from coronal, sagittal, and axial scans. The T1 and T2 maps showed comparable image quality in those time frames. Figure 4 shows the T1 and T2 maps from the other two volunteers, i.e., V2 and V3, using the sagittal scan. The SSIM of T1 and T2 maps from the proposed method were 0.61 and 0.75, respectively. The NRMSE for T1 and T2 maps (without considering vessel pixels) from the proposed method were 0.14 and 0.16, respectively. Figure 5 shows the preliminary 4D MRF scan on a volunteer, V4.Discussion
This study demonstrated a motion-resolved and free-breathing MRF with a 0.39-s/frame nominal resolution, i.e., 2.56 Hz for the nominal frame rate. The deformation maps were obtained from the MRF singular image. The iterative reconstruction method enforced the consistency of the MRF data with the deformation maps. Compared with the previous study on non-rigid motion correction for MRF that provided the motion-reduced images for one respiratory phase (3), the proposed one allowed the joint reconstruction of all time frames and provided the dynamic motion pattern of T1 and T2 maps.Conclusion
We demonstrated a motion-resolved and free-breathing MRF for liver imaging applications.Acknowledgements
The work was partly funded by the Hong Kong GRF fund (15102219) and the Hong Kong Health and Medical Research Fund (07182706, 06332916).References
1. Cruz G, Jaubert O, Schneider T, Botnar RM, Prieto C. Rigid motion-corrected magnetic resonance fingerprinting. Magn. Reson. Med. 2019;81:947–961 doi: 10.1002/mrm.27448.
2. Xu Z, Ye H, Lyu M, et al. Rigid motion correction for magnetic resonance fingerprinting with sliding-window reconstruction and image registration. Magn. Reson. Imaging 2019;57:303–312 doi: 10.1016/j.mri.2018.11.001.
3. Gastao Cruz, Olivier Jaubert, Haikun Qi, Torben Schneider, René M. Botnar and CP. Generalised Low-Rank Non-rigid Motion Corrected reconstruction for 3D free breathing Liver Magnetic Resonance Fingerprinting. In: ISMRM 2020. Paris; 2020. p. 3737.
4. Hamilton JI, Jiang Y, Chen Y, et al. MR fingerprinting for rapid quantification of myocardial T1, T2, and proton spin density. Magn. Reson. Med. 2017;77:C1 doi: 10.1002/mrm.26668.
5. Jaubert O, Arrieta C, Cruz G, et al. Multi-parametric liver tissue characterization using MR fingerprinting: Simultaneous T1, T2, T2*, and fat fraction mapping. Magn. Reson. Med. 2020;84:2625–2635 doi: 10.1002/mrm.28311.
6. Chen Y, Jiang Y, Pahwa S, et al. MR fingerprinting for rapid quantitative abdominal imaging. Radiology 2016;279:278–286 doi: 10.1148/radiol.2016152037.
7. Li T, Cui D, Hui ES, Cai J. Time-resolved magnetic resonance fingerprinting for radiotherapy motion management. Med. Phys. 2020;47:6286–6293 doi: 10.1002/mp.14513.
Figures