4182
Aberrant Spontaneous Brain Activity in Coronary Heart Disease: A Preliminary Resting-State Functional MRI Study.1Xiamen Cardiovascular Hospital of Xiamen University, Xiamen, China, 2GE Healthcare, Beijing, China
Synopsis
Coronary heart disease is an urgent, rapidly-developing disease with high disability and mortality. Previous studies indicated that CHD patients exhibited an increased risk of mild cognitive and emotional dysfunction. Here we collected rs-fMRI data to assess global brain activity in CHD patients and controls by measuring fALFF. We demonstrated that CHD patients occur abnormal brain activity in left precentral/postcentral gyrus and right inferior cerebellum, which is mainly related to sensorimotor network and pain processing. Spontaneous brain activity abnormalities may contribute to understanding underlying neurological mechanisms of CHD.
Introduction
Coronary heart disease (CHD), one of the primary causes of death, poses serious threats to physical health 1. Recent researches indicated that CHD had an increased risk of cognitive and emotional dysfunction 2. Hence, the focus of recent research turned to the brain function disorder caused by CHD. Many previous MRI studies have demonstrated that CHD has grey matter atrophy, white matter lesion, and cerebral blood flow change 3-7. However, little is known about the changes of CHD on brain activity. Resting-state functional MRI (rs-fMRI) is a noninvasive method to investigate the brain activity 8. Bernard et al. used rs-fMRI to study alterations of functional connectivity in patients with acute coronary syndrome and showed evidence of abnormal neural networks 9. This research focused on the alteration of functional connectivity between two aberrant brain areas. While the influence of CHD on the brain might be global, it is of great significance to analyze the whole brain activity in patients with CHD. The fractional amplitude of low‑frequency fluctuations (fALFF) enables measurement of the amplitude of spontaneous low-frequency BOLD signals, and offers valuable information about global brain activity 10. This study aimed to explore spontaneous brain activity changes by fALFF, and investigate their relationship with clinical characteristics in patients with CHD.Materials and Methods
The experiment was authorized by the Ethics Committee of Cardiovascular Hospital of Xiamen, and written informed consents were obtained from all participants. 25 patients with coronary heart disease and 35 age, gender, and education level-matched control subjects were included in this study. All MRI data were obtained using a 3-Tesla MRI scanner (SIGNA Pioneer, GE Healthcare, Milwaukee, WI). High-resolution structural images were acquired using a 3D T1-weighted inversion recovery prepared fast spoiled gradient-recalled echo (IR FSPGR) pulse sequence with following parameters: TE = 3.2 ms; TR = 8.3 ms; TI = 450 ms; FA = 12°; FOV = 240 mm × 240 mm; thickness = 1.0 mm; matrix = 240 × 240. Resting-state fMRI images, including 185 volumes, were acquired by a 2D gradient-recalled echo echo-planar imaging (GRE-EPI) pulse sequence in the axial plane with following parameters: TE = 30 ms; TR = 2000 ms; FA = 90°; FOV = 240 mm × 240 mm; thickness = 4.0 mm; matrix = 64 × 64. Voxel-based morphometry analysis was conducted based on the T1-weighted images using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm). fMRI data were first preprocessed by CONN software (www.nitrc.org/projects/conn) with slicing timing correction, motion correction, normalization, smoothing, and nuisance covariate regression. After preprocessing, the gray matter volume (GMV) and fALFF values were calculated. Differences in GVM and fALFF values between CHD and control groups were compared by independent two-sample t-test using SPM8 software (http://www.fil.ion.ucl.ac.uk./spm/). The cluster-level family-wise error (FWE) method was applied for multiple comparison correction, and a cluster-defined threshold was set to 0.001 and a corrected cluster significance was p < 0.05. Correlation analyses between the mean fALFF values and clinical characteristics were further assessed in CHD patients. In addition, receiver operating characteristic curves were conducted to access the diagnostic ability of the fALFF values.Results
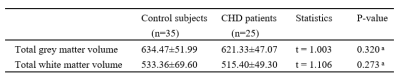
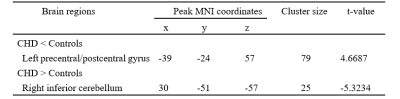
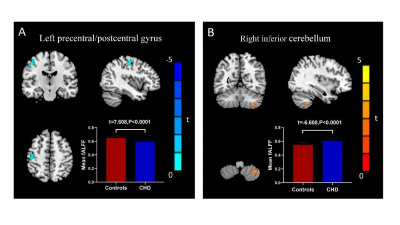
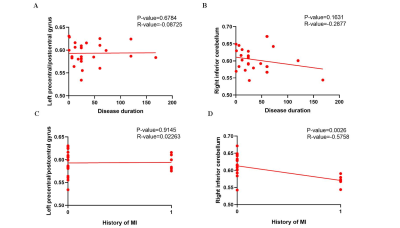
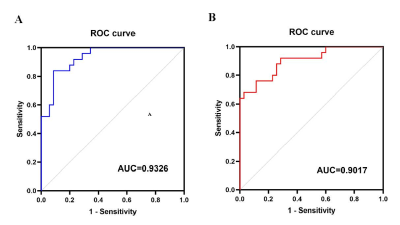
For the VBM analysis, while the GMV in CHD patients was smaller than that in the control group, no statistical difference was found. Details about total grey and white matter volume are shown in Table 1. Compared with the control group, patients with CHD showed decreased fALFF values in the left precentral/postcentral gyrus and increased fALFF values in the right inferior cerebellum (Figure 1 and Table 2). The fALFF values of the right inferior cerebellum were significantly lower in CHD patients with a history of MI. No statistical difference was discovered between the fALFF values of the left precentral/postcentral gyrus and the history of MI. There was also no significant correlation between the aberrant fALFF values and disease duration (Figure 2). We speculated that the significant differences in fALFF values might be helpful imaging biomarkers to differentiate CHD patients from controls, thus we further performed ROC curve analysis with the averaged fALFF values in three altered brain regions. The AUC results of mean fALFF values in the left precentral/postcentral gyrus and right inferior cerebellum were 0.9326 and 0.9017, respectively, indicating a good accuracy (Figure 3).Discussion and Conclusion
In this study, brain activity was measured via rs-fMRI in CHD patients and control group. Our investigation demonstrated that patients who suffer from CHD occur abnormal brain activity in the left precentral/postcentral gyrus and the right inferior cerebellum, which is mainly related to sensorimotor network and pain processing. Spontaneous brain activity abnormalities may contribute to understanding the underlying neurological mechanism of CHD. These findings lay a foundation for further study of the neurological mechanism of CHD.Acknowledgements
We thank all participants in this study.References
1. Wang HD, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388(10053): 1459-544.
2. van de Vorst IE, Koek HL, de Vries R, Bots ML, Reitsma JB, Vaartjes I. Effect of Vascular Risk Factors and Diseases on Mortality in Individuals with Dementia: A Systematic Review and Meta-Analysis. Journal of the American Geriatrics Society 2016; 64(1): 37-46.
3. Anazodo UC, Shoemaker JK, Suskin N, Ssali T, Wang DJJ, St Lawrence KS. Impaired Cerebrovascular Function in Coronary Artery Disease Patients and Recovery Following Cardiac Rehabilitation. Frontiers in Aging Neuroscience 2016; 7.
4. Santiago C, Herrmann N, Swardfager W, et al. White Matter Microstructural Integrity Is Associated with Executive Function and Processing Speed in Older Adults with Coronary Artery Disease. American Journal of Geriatric Psychiatry 2015; 23(7): 754-63.
5. Barekatain M, Askarpour H, Zahedian F, et al. The relationship between regional brain volumes and the extent of coronary artery disease in mild cognitive impairment. Journal of Research in Medical Sciences 2014; 19(8): 739-45.
6. Almeida OP, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Flicker L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. European Heart Journal 2012; 33(14): 1769-76.
7. Cui Y, Jiao Y, Chen YC, et al. Altered Spontaneous Brain Activity in Type 2 Diabetes: A Resting-State Functional MRI Study. Diabetes 2014; 63(2): 749-60.
8. Peterson A, Thome J, Frewen P, Lanius RA. Resting-State Neuroimaging Studies: A New Way of Identifying Differences and Similarities Among the Anxiety Disorders? Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie 2014; 59(6): 294-300.
9. Bernard C, Catheline G, Dilharreguy B, et al. Cerebral changes and cognitive impairment after an ischemic heart disease: a multimodal MRI study. Brain Imaging and Behavior 2016; 10(3): 893-900. 10. Zou QH, Zhu CZ, Yang YH, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. Journal of Neuroscience Methods 2008; 172(1): 137-41.
Figures