4174
Characterization of Corticospinal Tract Injury along Fibers by Automated Fiber Quantification in Stroke1Shanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science, East China Normal University, Shanghai, China, 2Department of rehabilitation, Huashan Hospital, Fudan University, Shanghai, China, 3School of Psychology and Cognitive Science, East China Normal University, Shanghai, China
Synopsis
Previous DTI studies mainly detected partial ROIs or mean diffusion profiles of corticospinal tract (CST), few revealed changes along CST trajectory. We used Automated Fiber Quantification (AFQ) derived from DTI to investigate whether diffusion parameters changes were localized to its specific regions or spread throughout. The results showed AFQ traced out a clearer picture of the damaged CST along its trajectory in patients with hemiplegia after stroke. Furthermore, the severity of several damaged CST segments was correlated with motor scores, possibly providing more accurate and reliable "target" regions for assessing clinical prognosis and the efficacy of motor rehabilitation.
Introduction
The corticospinal tract (CST) is a major neural tract in the human brain for the regulation of voluntary movements, particularly for the fine movements of the hands1,2. An increasing number of neuroimaging studies have confirmed that the integrity of the CST is essential for the effective recovery of impaired motor function in stroke patients3-5.Automated fiber quantification (AFQ) is a DTI-based technique and provides a more sensitive method of measuring fine anatomical changes in white matter tracts than conventional fiber tractography6. AFQ can precisely localize the differential segmentation of fiber tracts, which makes it a more accurate and comprehensive method for fiber quantification. The characteristics along the length of the CST may be quite different and some pathological changes may occur in a localized area rather than the entire fiber tracts. In this study, we selected a group of patients with hemiplegia and utilized the AFQ method to investigate: ⑴ the morphological integrity of the bilateral CSTs and the diffusion metrics changes along the CST trajectory. ⑵ the differences of CST morphological integrity and diffusion metrics between the stroke group and healthy controls. ⑶ whether certain segments of the CST were more closely related to motor dysfunction of the stroke patients.
Materials and methods
The chronic stroke patients with motor dysfunction were diagnosed with right hemisphere stroke. The stroke lesions were located in the left subcortex, mainly involving the basal ganglia. A total of 38 stroke patients were included, and 42 age- and sex-matched healthy controls were also enrolled. The upper limb section of Fugl-Meyer assessment scores (FMAUL)7 was used to evaluate motor function of stroke patients 8,9.All subjects underwent MRI scanning on a Siemens 3T magnetic resonance imaging system (Trio System). The imaging sequence included a high-resolution T1 weighted structural image, acquired using magnetization prepared rapid gradient echo (MPRAGE) imaging sequence (sagittal position, TR = 1900ms, TE = 3.42ms, field of view = 240mm × 240mm, 192 slices with 0.5mm gap; slice thickness = 1 mm, matrix size = 256×256). The diffusion tensor data were sampled using single-shot plane echo imaging (EPI) sequence (transverse position, TR = 6100ms, TE = 110ms, field of view = 256mm×256mm, 40 slices with no gap, slice thickness = 3mm, 30 non-collinear diffusion sensitization directions at b = 1000s / mm² and b0 image).The pre-processed DTI images and 3D-T1 weighted images were fed into the AFQ software (version 1.2; Stanford University, California, USA). The automated calculation was carried out using MATLAB (version R2014a; the Math Works, Inc., Natick, Massachusetts, USA). By an automated ROI approach, the cerebral peduncle (CP) is first tracked as the starting ROI of fiber tracking, and the central sulcus (CS) and the projection to the motor cortex are identified as the second ROI. The fiber bundle passing through the two ROIs is CST. The tract profiles of FA and MD at 100 points between the two ROIs were evaluated for each subject.
The relationships between tract-specific diffusion properties and FMAUL scores were assessed using Spearman correlation coefficients. The corrected significant threshold for point-wise diffusion properties of the CST was P < 0.05 with FDR correction. Age, gender, illness duration, and the size of the lesion were listed as nuisance covariate in correlation analyses.
Results
AFQ displayed the morphological difference of the CST in stroke patients and healthy subjects (Fig.1).Compared with HCs, the FA of the ipsilesional CST in the stroke group was significantly reduced in the span ranging from 3-100 points (Fig.2A), accompanied by MD increase in the 8-100th points (Fig. 2D). As to the right CST, the FA had no significant difference between the two groups (Fig.2B), while the MD increased in the 71-76 and 87-100 points, respectively (Fig. 2E). In addition, compared with HCs, FAasy of the stroke group increased significantly in 3rd-100th points of CST (Fig.2C), whereas MDasy revealed regional decrease in the 2-5th, 8-11th, 15-19th, 34-40th, 51-54th, 61th, 62th, 65-100th points, respectively (Figure 2F).
The mean FAasy of the CST was negatively correlated with FMAUL (r=-0.435, P=0.038), see Fig.3A. Point-wise AFQ showed FAasy of the CST was significantly negatively correlated with FMAUL scores(P<0.05) in three separate consecutive segments (11-18th points, 40-45th points, 90-100th points, respectively), see Fig. 3B.
Discussion
In this study, stroke lesions were concentrated in the left basal ganglia regions, but the AFQ results showed that the FA reduction of the CST not only appeared at the lesion areas, but was widespread throughout their pathways, thus our results further confirmed that there is both prograde and retrograde neurodegeneration involving the whole ipsilesional CST in hemiplegia. Particularly, it showed significant negative correlations between point-wise FAasy and FMA-UL in three intermittent segments, which located nearly to the cerebral peduncle, stroke lesion and motor cortex anatomically, which could not be visualized by traditional DTI analytical methods. The close relations between the remote prograde and retrograde neurodegeneration of the three segments of CST and motor deficits are worthwhile to be noted and further investigated.Conclusion
AFQ displayed the segmental damage of CST along its trajectory. AFQ-based FAasy may be a potentially important reference index for the assessment of upper limb motor deficits in stroke patients.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.82102665, 81471651 and 11835003); the Shanghai Sailing Program (No.21YF1404600)References
1. Jang, S.H., The corticospinal tract from the viewpoint of brain rehabilitation. Journal of rehabilitation medicine, 2014. 46(3): p. 193-199.
2. Cho, H.M., et al., The clinical characteristics of motor function in chronic hemiparetic stroke patients with complete corticospinal tract injury. NeuroRehabilitation (Reading, Mass.), 2012. 31(2): p. 207-213.
3. Jang, S.H., The role of the corticospinal tract in motor recovery in patients with a stroke: A review. NeuroRehabilitation (Reading, Mass.), 2009. 24(3): p. 285-290.
4. Byblow, W.D., et al., Proportional recovery after stroke depends on corticomotor integrity. Annals of neurology, 2015. 78(6): p. 848-859.
5. Stinear, C.M., et al., Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain (London, England : 1878), 2007. 130(1): p. 170-180.
6. Yeatman, J.D., et al., Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PloS one, 2012. 7(11): p. e49790-e49790.
7. van Wijck, F.M.J., et al., Assessing Motor Deficits in Neurological Rehabilitation: Patterns of Instrument Usage. Neurorehabilitation and neural repair, 2001. 15(1): p. 23-30.
8. Qiu, M., et al., White Matter Integrity Is a Stronger Predictor of Motor Function Than BOLD Response in Patients With Stroke. Neurorehabilitation and neural repair, 2011. 25(3): p. 275-284.
9. Langhorne, P.P., J.P. Bernhardt, and G.P. Kwakkel, Stroke rehabilitation. The Lancet (British edition), 2011. 377(9778): p. 1693-1702.
Figures

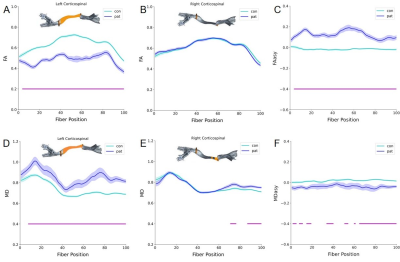
Fig.3. The correlations of and FAasy FMAUL in stroke patients. A: The mean FAasy of CST was negatively correlated with FMAUL (r=-0.435, P=0.038). B: The point-wise FAasy correlated with the FMAUL were mainly located at three separate consecutive segments of CST (11-18points, 40-45points, and 90-100points, respectively).