4155
Weakly-Supervised Carotid Vessel Wall Sub-pixel Segmentation Using Global-Local Context Aggregation and PolarMask1Center for Biomedical Imaging Research, Tsinghua University, Beijing, China, 2MSC Clinical & Technical Solutions, Philips Healthcare, Beijing, China
Synopsis
Carotid vessel wall segmentation on 3D black-blood MRI is a key step in plaque burden assessment and atherosclerotic lesions identification. In this study, a two-stage weakly-supervised carotid vessel wall segmentation approach was developed using limited manual delineations on 3D black-blood MR images. First, a global-local context aggregation strategy was used to identify bilateral carotid arteries robustly. Then, distances from artery center to boundaries were regressed in a polar coordinate by PolarMask to segment the vessel wall. The proposed segmentation approach outperformed Attention UNet (Quantitative score: 0.795±0.170 vs. 0.729±0.208) and showed great potential in quantitative atherosclerosis analysis.
Introduction
Carotid atherosclerosis is one of the leading causes of stroke worldwide1. 3D magnetic resonance vessel wall imaging (MR-VWI), such as 3D-MERGE (3D Motion Sensitized Driven Equilibrium prepared Rapid Gradient Echo)2, has shown great potential in carotid plaque analysis, benefitting from its large coverage and high isotropic resolution. However, its 3D characteristics cause complex review procedures, especially labor-intensive manual delineation of vessel wall. Here, we proposed a weakly-supervised deep learning approach for carotid vessel wall segmentation, which first utilized the shape-based mask interpolation to reduce the requirement of dense manual annotations, and then used the global-local context aggregation and PolarMask to perform sub-pixel vessel wall segmentation.Materials and Methods
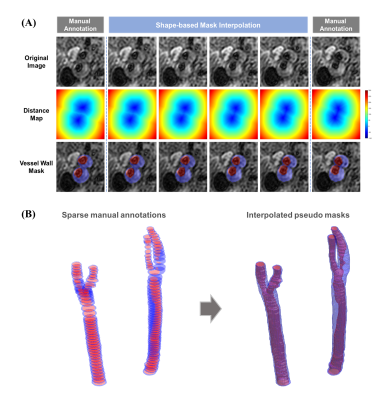
Study Population and Data ReviewThe datasets (training set: 25 subjects, test set: 25 subjects) were selected from the Carotid Atherosclerosis Risk Assessment (CARE II) study3, which enrolled Chinese patients with recent ischemic stroke or transient ischemic attack. With informed consent, each patient underwent the 3D-MERGE sequence acquisition. Vessel walls of bilateral common, internal, and external carotid arteries (CCA, ICA, and ECA) were manually annotated using a dedicated software (CASCADE, UW, Seattle, Washington, USA)4 by experienced radiologists. To reduce the workload of reviewers, about one out of every 5 consecutive slices were annotated in the training set (2584 labeled slices), and slices from random locations were annotated in the test set (2412 labeled slices). Fig. 1B shows an example of sparse manual annotations.
Annotation-efficient Shape-based Mask Interpolation
We applied a shape-based interpolation strategy (Fig. 1) to generate the dense pseudo vessel wall labels from sparse manual annotations to perform the weakly supervised training. As shown in Fig. 1A, for images with manual annotations, distance transformation was performed on vessel wall masks to generate reference distance maps5. For images without annotations, distance maps were generated by interpolating the two nearest reference distance maps. Then, a threshold of 0 was applied on distance maps to generate the pseudo masks, which could be used as the dense pseudo annotations in the following carotid artery localization.
Carotid Artery Localization Using Global-Local Context Aggregation
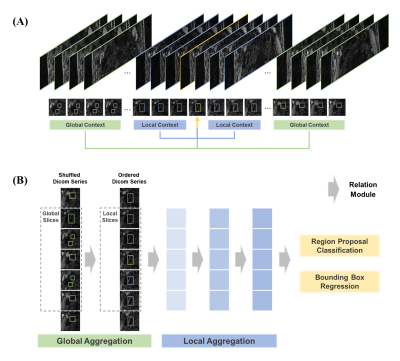
We adopted a two-stage segmentation strategy, in which the bounding box of the candidate carotid artery was located and then the vessel wall segmentation was performed. To better utilize the context information in the 3D image series, we applied a global-local context aggregation strategy6 to localize the carotid artery. Specifically, as shown in Fig. 2, the candidate bounding box was first generated by the region proposal network (RPN) on the current slice. Then, bounding boxes generated from adjacent slices and global randomly-selected slices constituted an auxiliary pool. Relation modules were applied to aggregate the candidate bounding box and local-global bounding boxes from the pool to generate the final bounding box containing the carotid artery.
Sub-pixel Vessel Wall Segmentation Based on PolarMask
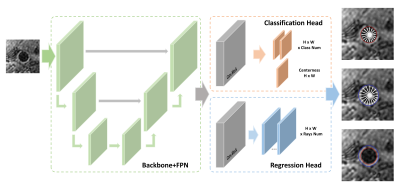
After locating the carotid artery, we used PolarMask7 to perform the carotid vessel wall segmentation. Notably, PolarMask generated the sub-pixel segmentation which could bring more accurate results. The inputs of PolarMask were image patches and the outputs were the distances from each artery center to the boundaries in the polar coordinate system. The PolarMask network (Fig. 3) consisted of a feature extraction network, a classification head for the differentiation of outer wall center or lumen center, and a regression head for the distances calculation. In this study, a fixed angle (10°) and 36 contour points predicted clockwise were used for contour regression. Then, the points were converted to smooth contours, i.e., sub-pixel vessel wall segmentation, using the B-spline interpolation.
Statistical Analysis
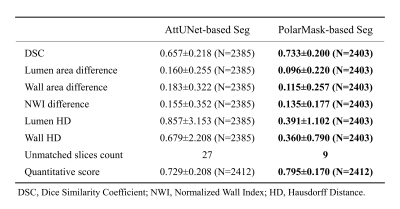
Results were quantitatively evaluated using: Dice Similarity Coefficient (DSC) of the vessel wall, lumen area difference, wall area difference, Normalized Wall Index (NWI) difference, lumen Hausdorff Distance (HD), wall HD and number of unmatched slices. Moreover, a quantitative score (QuanM) was calculated as weighted sum of all measurements: DSC (50%), lumen area (12.5%) and wall area (12.5%), NWI (12.5%), HD (12.5%). Attention UNet (AttUNet)8 was used as the compared method.
Results
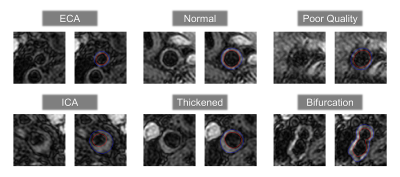
Our two-stage segmentation approach using PolarMask achieved a QuanM of 0.795±0.170 and a DSC of 0.733±0.200. Differences in lumen area, vessel wall area, and NWI between PolarMask-segmentation and manual annotations were 0.096±0.220, 0.115±0.257, and 0.135±0.177, respectively. HD between PolarMask-segmented and manual lumen and outer wall boundaries were 0.391±1.102 and 0.360±0.790, respectively. As a comparison, QuanM of AttUNet-segmentation was 0.729±0.208. PolarMask-based approach consistently performed better across all measurements (Table 1). The solution proposed in this study achieved robust carotid vessel wall segmentation results under different conditions, including ECA, ICA, normal vessel wall, thickened vessel wall, poor-quality image, and bifurcation (Fig. 4).Discussion and Conclusion
In this study, a weakly-supervised deep learning approach was proposed to segment the carotid vessel wall, in which the shape-based mask interpolation was first utilized to reduce the requirement of dense manual annotations, and then the global-local context aggregation and PolarMask were used to perform the sub-pixel carotid vessel wall segmentation. The proposed approach avoided the dense manual annotations and utilized the local and global context information of 3D data, achieving robust identification of bilateral carotid arteries and sub-pixel segmentation of vessel wall, which may provide a promising tool for quantitative carotid atherosclerosis analysis in large population studies.Acknowledgements
None.References
1. Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44:3071-7.
2. Balu N, Yarnykh VL, Chu BC, Wang JN, Hatsukami T, Yuan C. Carotid Plaque Assessment Using Fast 3D Isotropic Resolution Black-Blood MRI. Magn Reson Med 2011;65:627-37.
3. Zhao X, Li R, Hippe D S, et al. Chinese Atherosclerosis Risk Evaluation (CARE II) study: a novel cross-sectional, multicentre study of the prevalence of high-risk atherosclerotic carotid plaque in Chinese patients with ischaemic cerebrovascular events—design and rationale[J]. Stroke and vascular neurology, 2017, 2(1).
4. Kerwin W, Xu D, Liu F, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis[J]. Topics in Magnetic Resonance Imaging, 2007, 18(5): 371-378.
5. Raya S P, Udupa J K. Shape-based interpolation of multidimensional objectsJ. IEEE transactions on medical imaging, 1990, 9(1): 32-42.
6. Chen Y, Cao Y, Hu H, et al. Memory enhanced global-local aggregation for video object detection[C]//Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition. 2020: 10337-10346.
7. Xie E, Sun P, Song X, et al. Polarmask: Single shot instance segmentation with polar representation[C]//Proceedings of the IEEE/CVF conference on computer vision and pattern recognition. 2020: 12193-12202.
8. Oktay O, Schlemper J, Folgoc L L, et al. Attention u-net: Learning where to look for the pancreas[J]. arXiv preprint arXiv:1804.03999, 2018.
Figures