4154
Reduced coupling between global blood-oxygen-level-dependent signal and cerebrospinal fluid inflow is related to small vessel disease1The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, 2GE Healthcare, Shanghai, China
Synopsis
The association between the glymphatic dysfunction and cerebral small vessel disease (SVD) is still unclear due a lack of in vivo measures. In this work, we employed a novel method based on the coupling between global blood-oxygen-level-dependent (gBOLD) signal and cerebrospinal fluid (CSF) inflow, which could detect alterations in CSF dynamics. We found that patients with severe SVD had significantly impaired glymphatic function. The dilation of peri-vascular space and the presence of diabetes were associated with worse glymphatic function. These results may provide useful knowledge for understanding the mechanism of SVD and improving clinical treatment.
Introduction
Glymphatic dysfunction may participate in the initiation and progression of cerebral small vessel disease (SVD)1-3. Currently, the association between glymphatic dysfunction and SVD has not been fully demonstrated in human due to a lack of in vivo measures. Specifically, which vascular risk factor or brain imaging marker was associated with impaired glymphatic function, and their causal relationships are not clear.Decreases in driving forces may impair glymphatic clearance. While the effect of cardiac pulsatility on cerebrospinal fluid (CSF) inflow has been well confirmed4, fluctuations in global cerebral blood flow (CBF) due to low-frequency (<0.1Hz) intrinsic vasomotor contractions or neural activities may produce much larger forces5, 6. This phenomenon could be detected in vivo by measuring the coupling between global CBF (reflected by global blood-oxygen-level-dependent signal, gBOLD) and CSF inflow (reflected by inflow-related image enhancement)7. Studies confirmed that this gBOLD-CSF coupling exists either during sleep or wakefulness6, 7. Furthermore, altered coupling was found closely associated with pathological markers and clinical functions in patients with Alzheimer’s disease8 and Parkinson’s disease9, suggesting its potential as a marker of glymphatic function.
In the present study, we aim to investigate: (1) the association between SVD severity (including specific vascular factors) and glymphatic dysfunction measured by gBOLD-CSF coupling; (2) the relationship between the gBOLD-CSF coupling and cognitive impairments.
Methods
The study protocols were approved by the ethics committee of the 2nd Affiliated Hospital, Zhejiang University School of Medicine. All participants signed informed consent on admission.One hundred and forty-six SVD patients underwent a 3T MRI examination (acquiring T1-weighted (T1W) images, T2 fluid attenuated inversion recovery (T2 FLAIR) images,susceptibility-weighted (SWI) images and resting-state functional MRI (rsfMRI) images). All participants signed informed consent on admission. The demographic and clinical information were collected, and the subjects were required to complete a set of neuropsychological assessments covering global cognition, executive function and short-term memory.
We evaluated the SVD imaging markers according to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE), including white matter hyperintensity (WMH), lacunes, microbleeds (CMBs) and perivascular space (PVS) in the basal ganglia (BG) and centrum semiovale (CSO) regions.
Consistent with previous studies7-9, the gBOLD signal was extracted from cortical gray matter, and CSF inflow signal was extracted frome the bottom slices of fMRI images (near the bottom of the cerebellum), because they were most sensitive to the CSF inflow effect7. We manually drew the CSF ROIs on functional images, and its anatomic location was further confirmed on T1 images which were co-registered with functional images (Figure 1). We set the BOLD signal as reference and calculated the maximal cross-correlation between the gBOLD signal and CSF inflow over a range time lags (-20s ~ 20s) for each patient. Besides this, the cross-correlation function between the negative derivative of the global BOLD signal and CSF signal were also calculated for demonstrating that the oscillation of cerebral blood volume would lead to the CSF dynamics. Finally, we verified the credibility of the above cross-correlation between BOLD and CSF signals by permutation test. To help better understand the result, the opposite of gBOLD-CSF coupling----Couplinginv----was calculated and used in statistical analyses. Bigger Couplinginv indicates better glymphatic function. Head motion (reflected by framewise displacement) and arousal state (reflected by the standard deviation of the gBOLD signal were calculated and introduced as covariates.
We performed correlation analyses to explore the relationships between the confounders and Couplinginv. Multiple linear regression models were employed to investigate the associations between the Couplinginv and vascular factors, as well as cognitive scores.
Result
The median age was 67 years, and 66 (45.2%) were female. The median educational year was 8. In general, the subjects had moderate-to-severe SVD. Correlations between Couplinginv and head motion \arousal state were not significant. Couplinginv was negatively correlated with the age (r = -0.187, p = 0.02).Lower Couplinginv was associated with severer SVD (β = -0.177, p = 0.037). The dPVS (β = -0.165, p = 0.042) and diabetes (β = -0.181, p = 0.026) were associated with reduced gBOLD-CSF coupling. Stronger coupling was significantly related to better Digit Symbol Substitution Test performance (β = 0.147, p = 0.039).
Discussion
In this study, we found that severe SVD patients had reduced gBOLD-CSF coupling, indicating alteration in CSF dynamics supporting the glymphatic function. This provides evidence regarding functional impairments of the glymphatic system, in addition to PVS structural changes in SVD.Among all the SVD imaging markers, reduced coupling was associated with BG-PVS dilation, suggesting coherent structural-functional changes. It might be due to that the vacuum effect induced by vasoconstriction decreased, and directional flow turned into turbulence in large dPVS. The diabetes could damage vascular smooth muscle cells, leading to impaired vasomotor and decreased CSF driving forces, yielding lower gBOLD-CSF coupling.
Our findings may provide useful knowledge for understanding the mechanism of SVD and improving clinical treatment. Futures studies in larger cohorts encompassing different stages of SVD are needed to validate these results.
Acknowledgements
This study was supported by the 13th Five-year Plan for National Key Research and Development Program of China, the National Natural Science Foundation of China, the Natural Science Foundation of Zhejiang Province, and the China Postdoctoral Science Foundation.References
1. Zhang W, Zhou Y, Wang J, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage 2021;238:118257.
2. Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 2017;131:2257-2274.
3. Benveniste H, Nedergaard M. Cerebral small vessel disease: A glymphopathy? Curr Opin Neurobiol 2021;72:15-21.
4. Hablitz LM, Plá V, Giannetto M, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun 2020;11:4411.
5. Helakari H, Korhonen V, Holst S, et al. Sleep-specific changes in physiological brain pulsations. bioRxiv 2020:2020.2009.2003.280479.
6. Yang H-C, Inglis B, Talavage TM, et al. Coupling between cerebrovascular oscillations and CSF flow fluctuation during wakefulness: An fMRI study. bioRxiv 2021:2021.2003.2029.437406.
7. Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019;366:628-631.
8. Han F, Chen J, Belkin-Rosen A, et al. Reduced coupling between cerebrospinal fluid flow and global brain activity is linked to Alzheimer disease-related pathology. PLoS Biol 2021;19:e3001233.
9. Han F, Brown GL, Zhu Y, et al. Decoupling of Global Brain Activity and Cerebrospinal Fluid Flow in Parkinson's Disease Cognitive Decline. Mov Disord 2021;36:2066-2076.
Figures
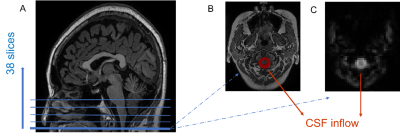
Figure 1. The region for extracting CSF signal. (A) Example of scanning localization. The functional images were acquired from bottom to top (interleaved), with a total of 38 slices covering the whole brain. (B) and (C) were the bottom slice of the axial T1W and rsfMRI images. The red area was used to extract CSF signal.
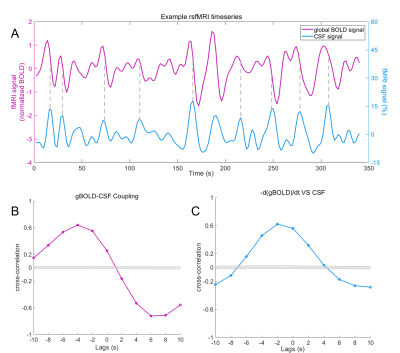
Figure 2. Example of gBOLD-CSF coupling. (A) CSF inflow peaks often appeared during the fast-declining period of the gBOLD signal (dashed vertical lines). (B) The cross-correlation between gBOLD and CSF signals was strongest at around 6s. (C) The negative derivative of gBOLD signal was also closely coupled to the CSF signal. The maximum was around -2s. Gray dashed line in (B) and (C) represent 95% confidence interval of the null distribution. gBOLD signal: global blood-oxygen-level-dependent signal.