4151
Middle Cerebral Artery Morphologies Associated with White Matter Hyperintensities1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Neurology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai, China, 3Human Phenome Institute, Fudan University, Shanghai, China
Synopsis
The current report summarizes the morphological characteristics of middle cerebral artery quantified from MRA scans in a large sample of older Chinese population and shows that higher M1 radius is an independent predictor of white matter hyperintensities (WMH). Furthermore, as the pathological changes resulting in white matter lesions are heterogeneous, the current study suggest that the spatial distribution patterns of WMH could reflect the underpinning pathological mechanisms, though further studies are required.
Introduction
Changes of large cerebral artery morphologies quantified from brain MRA, the most commonly adopted measurement for cerebral small vessel disease in clinical routines, have been found to associate with white matter hyperintensities (WMH) 1, 2-4. It is not clear whether morphological changes in critical cerebrovascular branches, such as the middle cerebral artery (MCA), are also associated with small vessel lesions. In order to corroborate the intrinsic association between the large and small vessels, we selected the M1 segment of the MCA, a critical intracranial artery branch which has occupied nearly half of the blood flow into brain 5, as the target branch and analyzed the correlation between its morphology and white matter lesions.Methods
This study retrospectively enrolled 2739 participants, free of acute stroke and large artery stenosis from Yueyang hospital, Shanghai, China. Brain MRI scans were completed on one 3T MR scanner (Philips). Figure 1 summarized the participants characteristics. The MRI protocol included T1-weigthed imaging (TE = 2.3ms, TR = 250ms, flip angle = 75°, matrix size = 512×512×18, voxel size = 0.45mm×0.45mm×6mm), T2-FLAIR (TE = 120ms, TR = 7000ms, flip angle = 90°, matrix size = 384×384×18, voxel size = 0.6mm×0.6mm×6mm) and TOF MRA (TE = 3.5ms, TR = 23ms, flip angle = 18°, matrix size = 560×560×112, voxel size = 0.375mm×0.375mm×0.8mm). This study was approved by the local ethics committee and informed consent was obtained for all participants. WMH lesions were segmented automatically by the lesion prediction algorithm in the LST toolbox (www. statistical-modelling.de/lst.html) from T2-FLAIR images. For each individual, T2-FLAIR images were registered to the T1-weighted images using an affine transformation and the T1-weighted images were registered to the MNI brain atlas with voxel size = 1mm×1mm×1mm by non-linear transformation. Then, two transformations were concatenated to transform the T2-FLAIR images and corresponding WMH lesions images into the reference space. WMH lesions were divided into periventricular WMH or deep WMH according to the distance from the lateral ventricles (>10 mm was considered to be deep WMH). Cerebral vessel segmentation was completed on TOF MRA images using the previously described methods1. The vessels were then reconstructed into the 3D space to allow interactive manual selection of start and end points of the first segment (M1) of the MCA. The morphological features and the centerline information of the MCA were calculated based on the segments between each pair of start and end points. Linear regression analysis was used to examine the associations of WMH with vascular risk factors and M1 morphological features. Logarithmic transformation was applied to reduce the skewness of the distribution of WMH volume (WMHV). Voxel-wise general linear regression with a logistic link function was used to estimate the associations between WMH frequency and vascular risk factors6.Results and Discussion
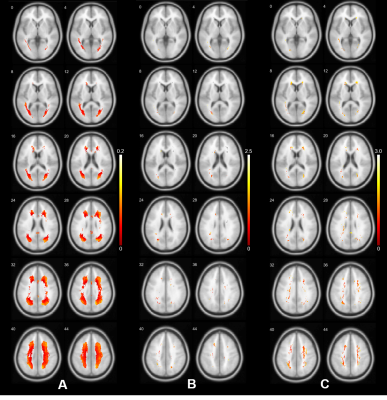
The associations of WMH with vascular risk factors and MCA morphologies are presented in Figure 2. Age was the most significant predictor of WMH volume. No significant correlations between vascular risk factors and WMHV was found, presumably due to the predominate effect of age given the mean age of our sample was 68.6 (11.0) years. Hypertension was associated with increased WMHV after adjusting for age. Increased M1 radius, length and tortuosity were all significantly correlated with increased total WMHV in the bivariable model. In the multivariable model adjusting for the predefined vascular risks, MCA radius and length remained significantly correlated with WMHV, suggesting morphological alternations of the MCA could still influence WMH, independent of age and hypertension. Figure 3 shows the voxel-wise spatial distribution of WMHs significantly associated with each vascular risk (age, hypertension, and MCA radius for Fig.3.A, B and C respectively). Age was found to be associated with perfused WMHs, whereas the WMHs associated with the other two were predominately periventricular.Conclusion
In conclusion, we found that the several structural alternations of the M1 segment could significantly predict the severity of WMH (indicated by volume). Our study presents evidence for differential spatial patterns of WMHs associated with different risk factors in a relatively large population. Although a causal relationship between altered MCA morphology cannot be established based on current evidence, different spatial distribution patterns of WMHs could possibly suggest different pathological changes underpinning the lesions.Acknowledgements
No acknowledgement found.References
1.Zhang B, Wang Y, Wang B, et al. MRI-Based Investigation of Association Between Cerebrovascular Structural Alteration and White Matter Hyperintensity Induced by High Blood Pressure. Journal of Magnetic Resonance Imaging 2021.
2.Habes M, Erus G, Toledo JB, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016;139:1164-1179.
3.ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral small vessel disease: from a focal to a global perspective. Nature Reviews Neurology 2018;14(7):387-398.
4.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurology 2019;18(7):684-696.
5.Zarrinkoob L, Ambarki K, Wahlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. Journal of Cerebral Blood Flow and Metabolism 2015;35(4):648-654.
6. Rostrup E, Gouw AA, Vrenken H, et al. The spatial distribution of age-related white matter changes as a function of vascular risk factors-Results from the LADIS study. Neuroimage 2012;60(3):1597-1607.
Figures

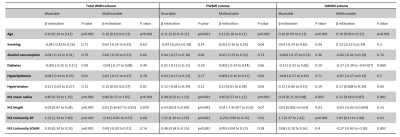
Figure 2. Association of white matter hyperintensities with vascular risk factors and middle cerebral artery morphology features.M1, M1 segment of the middle cerebral artery. WMH, white matter hyperintensities. PWMH, periventricular white matter hyperintensities. DWMH, deep white matter hyperintensities. DF, distance factor. SOAM, sum of angle metrics.