4144
Vulnerable plaque characteristics on MR vessel wall imaging predict hemodynamic instability during carotid artery stenting1Department of Ultrasound, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China, 2Department of Vascular Surgery, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China, 3Department of Radiology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China, 4Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University School of Medicine, Beijing, China
Synopsis
It has been shown that about 30%-70% of the patients underwent carotid artery stenting (CAS) suffered from perioperative hemodynamic instability (HI) characterized by transient hypotension and bradycardia. This study investigated the association between vulnerable plaque characteristics on MR vessel wall imaging and HI during CAS. We found that patients in HI group had significantly larger wall area and total vessel area, higher prevalence of intraplaque hemorrhage and vulnerable plaque, and larger volume of lipid-rich necrotic core (LRNC) of carotid plaque compared to those in non-HI group. The volume of LRNC and presence of vulnerable plaque could effectively predict HI.
Introduction
Carotid artery stenotic disease is one of the major etiologies of ischemic stroke1. Carotid artery stenting (CAS) is a common treatment for carotid artery stenosis2. However, 30%-70% of the patients underwent CAS suffered from perioperative transient hypotension and bradycardia, which is defined as hemodynamic instability (HI)3. This phenomenon will lead to decline in cognitive function and subsequent cerebrovascular dementia4. To predict HI before CAS surgery is challenging due to limited evidences in the influence factors and mechanism of HI.HI might be a sequela of sinus reflex due to the stenting procedures and the interaction between stent and atherosclerotic plaques might be related to the extent of sinus reflex5. Previous studies have demonstrated carotid plaque morphology and compositions were associated with HI6-8. MR vessel wall imaging has been proved to be an ideal approach in characterizing carotid vulnerable plaque compositions9-11. However, few studies utilized MRI to assess the relationship between carotid vulnerable plaque features and perioperative HI.
This study aimed to investigate the association between the characteristics of carotid plaques determined by MR vessel wall imaging and perioperative HI in patients with moderate-to-severe carotid artery atherosclerotic stenosis referred to CAS surgery.
Methods
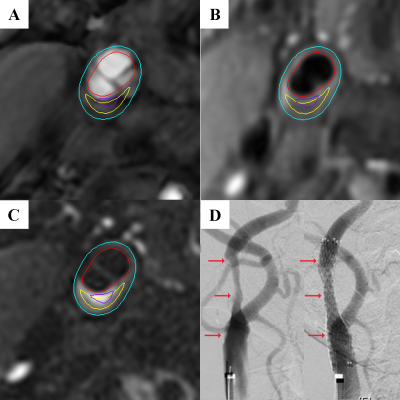
Study sample: Patients with carotid stenosis who were referred to CAS were recruited and underwent carotid artery MR vessel wall imaging at baseline. Clinical data were collected from patients’ medical records. The study protocol was approved by local ethnics committee and all patients provided written consent form. MR imaging: All patients underwent carotid artery plaque imaging on a 3.0T MR scanner (Discovery 750, GE Healthcare, Milwaukee, USA) using a carotid artery 8-channel phased array surface coil. The imaging sequences and parameters were as follows: 3D TOF: TR/TE 21/3.3 ms, flip angle 30°; 2D black-blood T1WI: FSE, DIR, TR/TE 800/10 ms; 2D black-blood T2WI imaging: FSE, DIR, TR/TE 4412/103 ms. All sequences were acquired with the same FOV of 140 mm x 140 mm and slice thickness of 2 mm and the longitudinal coverage was 32 mm centered to carotid bifurcation. Image analysis: Two experienced radiologists utilized a custom-designed software (CASCADE; University of Washington, Seattle) to analyze MR images. Lumen and wall boundaries of carotid arteries were outlined manually to measure lumen area, wall area, total vessel area, and wall thickness axially. Calcification, lipid-rich necrotic core (LRNC), intraplaque hemorrhage (IPH), and fibrous cap rupture were identified and quantified using the published criteria12,13. Vulnerable plaque was defined as plaques with IPH, fibrous cap rupture or large LRNC (area of LRNC / vessel wall >40%). Evaluation of HI: During CAS surgery, blood pressure was recorded before pre-dilation and every minute for 10 minutes after the stent implantation via upper arm cuff blood pressure monitoring. The blood pressure drop (BPD) was defined as the minus between the systolic blood pressure (SBP) before pre-dilation and the lowest SBP after stent implantation. The HI was defined as BPD ≥30 mmHg or the lowest SBP <90 mmHg. Statistical analysis: The characteristics of carotid plaques were compared using independent t test, Mann-Whitney U test, Chi-square test or Fisher exact test between the HI and non-HI group. The association between carotid plaque characteristics and HI was analyzed using logistic regression.Results
Of 56 recruited patients (mean age: 68.7±8.3 years; 44 males), 8 (14%) had history of stroke, 46 (82%) had hypertension, 17 (30%) had diabetes, and 20 (36%) had cardiovascular disease. Patients with HI (26, 46%) had significantly larger wall area (44.9±12.9 mm2 vs 36.9±7.5 mm2; p=0.008) and total vessel area (79.7±17.2 mm2 vs 69.9±17.3 mm2; p=0.031), higher prevalence of IPH (62% vs 30%; p=0.018) and vulnerable plaque (77% vs 43%; p=0.011), and larger volume of LRNC (344.7 [155.1, 665.7] mm3 vs 103.1 [53.9, 162.9] mm3; p=0.001) in carotid plaques compared to those without HI (30, 54%). Univariate regression analysis showed that carotid wall area (OR, 1.098; 95% CI, 1.022-1.180; p=0.011) and total vessel area (OR, 1.035; 95% CI, 1.001-1.070; p=0.045) were significantly associated with HI. Maximum wall thickness showed marginal association with HI (p=0.054). Presence of IPH (OR, 3.733; 95% CI, 1.229-11.338, p=0.020), volume of LRNC (OR, 1.004; 95% CI, 1.001-1.007; p=0.005), presence of vulnerable plaque (OR, 4.359; 95% CI, 1.362-13.954; p=0.013) were found to be significantly associated with HI. After adjusting for age, sex and BMI, wall area (p=0.044), volume of LRNC (p=0.014) and presence of vulnerable plaque (p=0.027) remained significant association with HI. After further adjusting for plaque burden of NWI, only the associations of volume of LRNC (p=0.013) and presence of vulnerable plaque (p=0.058) with HI showed statistically and marginally significant, respectively (Figure).Discussion & Conclusion
This is the first study to investigate the relationship between carotid plaque characteristics and HI utilizing MR vessel wall imaging. We found that carotid vulnerable plaque features such as lipid-rich lesions were significantly associated with HI. The atherosclerotic plaque with LRNC will transmit the pressure of stent implantation to a wide range of intima, thus stimulates large number of neuroreceptors, which finally leads to a larger drop of blood pressure. Our data suggest that patients with larger plaque burden and vulnerable plaque features, particularly larger LRNC, on vessel wall MR imaging may have higher risk of developing HI during CAS procedure.Acknowledgements
Funding: This study is supported by grants from the Beijing Tsinghua Changgung Hospital Fund (no. 12016C1005 and 12017C1015) and the National Natural Science Foundation of China (81771825).References
1. Ntaios G, Hart RG. Embolic Stroke. Circulation. 2017;136(25):2403-2405.
2. Sardar P, Chatterjee S, Aronow HD, et al. Carotid Artery Stenting Versus Endarterectomy for Stroke Prevention: A Meta-Analysis of Clinical Trials. J Am Coll Cardiol. 2017;69(18):2266-2275.
3. Mylonas SN, Moulakakis KG, Antonopoulos CN, et al. Carotid artery stenting-induced hemodynamic instability. J Endovasc Ther. 2013;20(1):48-60.
4. Pancak J, Wagnerova H, Škultéty Szárazová A, et al. Multi-infarct dementia and Alzheimer disease, contribution of cerebral circulation ultrasonography to pathogenesis and differential diagnosis. Value of microembolisation. Neuro Endocrinol Lett. 2016;37(2):137-140.
5. Kikuta S, Iwanaga J, Kusukawa J, et al. Carotid Sinus Nerve: A Comprehensive Review of Its Anatomy, Variations, Pathology, and Clinical Applications. World Neurosurg. 2019;127:370-374.
6. Rubio G, Karwowski JK, DeAmorim H, et al. Predicting Factors Associated with Postoperative Hypotension following Carotid Artery Stenting. Ann Vasc Surg. 2019;54:193-199.
7. Saleh M, Ali H, Atalla K, et al. Predictors of Carotid Artery Stenting-Induced Hemodynamic Instability. Vasc Endovascular Surg. 2021;55(5):475-481.
8. Nonaka T, Oka S, Miyata K, et al. Prediction of prolonged postprocedural hypotension after carotid artery stenting. Neurosurgery. 2005;57(3):472-477.
9. Zhou Z, Li R, Zhao X, et al. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17(1):41.
10. Fan Z, Yu W, Xie Y, et al. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: technical development and clinical feasibility. J Cardiovasc Magn Reson 2014;16(1):53.
11. Li L, Chai JT, Biasiolli L, et al. Black-blood multicontrast imaging of carotid arteries with DANTE-prepared 2D and 3D MR imaging. Radiology 2014;273(2):560-569.
12. Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106(11):1368-1373.
13. Chu B, Kampschulte A, Ferguson MS, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke. 2004;35(5):1079-1084.
Figures