4140
Assessment of myocardial microstructural change using novel motion compensated technique in cardiac diffusion tensor imaging
Wataru Ueki1, Yoshiaki Morita1, Yu Ueda2, Masaru Shiotani1, Tatsuhiro Yamamoto1, Yasuhiro Nagai1, Yasutoshi Ohta1, Keizo Murakawa1, and Tetsuya Fukuda1
1National Cerebral and Cardiovascular Center, Suita, Japan, 2Philips Japan Ltd, Tokyo, Japan
1National Cerebral and Cardiovascular Center, Suita, Japan, 2Philips Japan Ltd, Tokyo, Japan
Synopsis
The accelerated motion compensation (aMC) using higher order motion compensated gradients with asymmetric bipolar diffusion waveform can improve the image quality of cardiac DTI compared with conventional method and provide the relatively constant DTI marker. In LVH patients, aMC-DTI derived possible preliminary insights into detection of abnormal changes in myocardial microstructures in-vivo, even in segments without LGE.
INTRODUCTION
Cardiac diffusion tensor imaging (DTI) allows for in vivo characterization of myocardial microstructure non-invasively and has a potential to detect the myocardial disarray in various myocardial diseases1,2, including ventricular hypertrophy, such as hypertrophic cardiomyopathy (HCM), hypertension, athlete heart and so on. However, application of DTI for the heart is challenging due to image quality degradation caused by cardiac and respiratory motion. Recently, novel technique, which is accelerated motion compensation (aMC) using higher order motion compensated gradients with asymmetric bipolar diffusion waveform (Fig.1), has been developed as less sensitive method for cardiac motion3,4 and is a useful approach for reduction of motion artifacts, resulting in improvement of the myocardial visualization. This study aimed to evaluate the feasibility of the aMC-DTI compared with conventional DTI in healthy volunteers, and to assess the usefulness for detection of myocardial abnormal microstructure in patients with ventricular hypertrophy.METHODS
Cardiac DTI was performed using a clinical 3T scanner (Ingenia Elition, R5.6.1.1,Philips,Best, the Netherlands). 1. Four healthy normal volunteers (4 male, mean age 25±1.6 years) underwent cardiac DTI (Spin echo EPI: 1.06x1.06x6mm3, 3 slices, b factor= 0/300 s/mm2, 6 directions) using aMC (TE=44msec) and conventional method (TE=87msec) at short-axial plane in basal, mid-ventricular and apical level with respiratory navigator tracking. In left ventricular septal wall, image quality of fiber tracking (visualization and continuity of myocardial fiber) in aMC and conventional DTI were evaluated using the 4-point scale (3=Excellent, 2=good, 1=fair, 0=poor). Heart rate (HR; bpm) were recorded as parameters related cardiac motion. The fractional anisotropy (FA) in each segment of septal wall of three short-axis images were also compared between two methods. 2. Nine patients with left ventricular hypertrophy (LVH; 4 HCM, 2 Hypertensive heart disease) underwent cardiac DTI using aMC technique. MD (Mean diffusivity) and FA in each septal segment were compared between patients and healthy normal volunteers. LGE (Late gadolinium enhancement) was also performed with IR-TFE using null point method after gadolinium administration. In LVH patients, the presence or absence of LGE was decided visually for each segment.RESULTS
1. aMC-DTI tended to be higher points in myocardial visibility scale compared with conventional DTI, especially, in high-HR group (cut off=70bpm), aMC-DTI showed significantly higher point (p>0.05, Figure2). Whereas there were no significant difference between aMC and conventional DTI in low-HR group. In the mean FA, there were no significant difference between aMC and conventional DTI (aMC: 0.48 vs. conventional DTI: 0.46, p>0.05, Figure3). The variation of FA in aMC-DTI was sufficiently small compared to the variation of conventional method (Figure3). 2. Patients with LVH had significantly higher MD (LVH: 2.16 vs. normal: 1.85, p<0.01, Figure4) and lower FA (LVH: 0.35 vs. normal: 0.4, p<0.01, Figure4) compared with healthy normal volunteers. Further, not only LGE positive but apparently LGE negative segments also showed higher MD and FA values than normal myocardium (MD| LGE positive: 2.26 vs. normal: 1.85 p<0.05, LGE negative: 2.1 vs. normal: 1.85 p<0.01, FA| LGE positive: 0.32 vs normal: 0.4 p<0.01, LGE negative: 0.36 vs. normal: 0.4 p<0.05).DISCUSSION
In the present study, the application of aMC can improve the image quality of cardiac DTI compared with conventional method, especially effective for high HR cases, and provide the relatively constant DTI marker. Further aMC technique is expected to also reduce the respiratory motion related artifact in navigator gating, such as due to irregular breathing pattern. Secondary order motion-compensated diffusion encoding shows a linear dependency of the helix angles as a function of transmural depth for all trigger delays and reduced variation along the ventricular circumference3. Therefore, this novel method decreases the sensitivity to cardiac motion, thereby enabling cardiac DTI over a wider range of time points during cardiac contraction. In this study, the improvement by using aMC-DTI in the myocardial visibility in lower HR group was not so much. Shorter TE in conventional DTI might be the cause of less image degradation. Further technical development as less sensitive motion compensated method for cardiac motion is desired. In patients with LVH, aMC-DTI depicts an increase in amplitude and isotropy of diffusion compared to normal myocardium, as reflected by increased MD and decreased FA values. Further, apparently LGE negative LVH showed higher MD and lower FA values than were seen in a normal myocardium, suggesting that aMC-DTI can detect the early abnormal change of microstructure which is difficult to detect using LGE reflected by myocardial fibrosis. Future work including the comparison with endomyocardial biopsy (i.e. histological collagen volume and arrangement) and the assessment of abnormal myocardial microstructure in larger patients with various myocardial diseases are needed to evaluate the robustness of aMC-DTI.CONCLUSION
For cardiac DTI, the application of aMC technique improved the visibility of myocardium and provide the relatively constant DTI marker compared with conventional DTI. In LVH patients, aMC-DTI derived possible preliminary insights into detection of abnormal changes in myocardial microstructures in-vivo.Acknowledgements
No acknowledgement found.References
- Laura-Ann McGill, Reproducibility of in-vivo diffusion tensor cardiovascular magnetic resonance in hypertrophic cardiomyopathy, McGill et al. Journal of Cardiovascular Magnetic Resonance 2012, 14:86 2.
- Arka Das, Acute Microstructural Changes after ST-Segment Elevation Myocardial Infarction Assessed with Diffusion Tensor Imaging, Radiology. 2021 Apr;299(1):86-96.doi: 10.1148/radiol.2021203208. Epub 2021 Feb 9.
- Christian T. Stoeck, Second-Order Motion-Compensated Spin Echo Diffusion Tensor Imaging of the Human Heart, Magnetic Resonance in Medicine 75:1669–1676 (2016)
- Sonia Nielles-Vallespin, Cardiac Diffusion: Technique and Practical Applications, J. MAGN. RESON. IMAGING 2020;52:348–368.
Figures
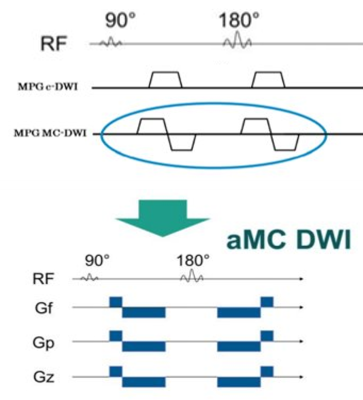
Pulse sequence diagram. This technique,
which is accelerated motion compensation (aMC) using higher order motion
compensated gradients with asymmetric bipolar diffusion waveform, has been
developed as less sensitive method for cardiac motion and is a useful approach
for reduction of motion artifacts, resulting in improvement of the myocardial
visualization.
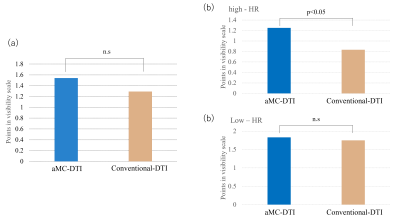
(a)Comparison of points in myocardial
visibility scale between aMC and conventional-DTI.
(b)Comparison of points in myocardial
visibility scale between aMC and conventional-DTI in high and low HR groups.
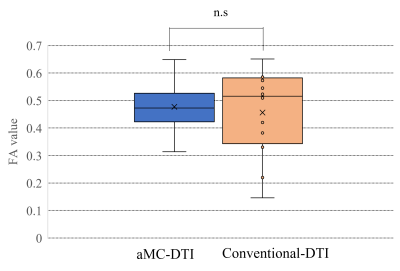
Comparison of FA value between aMC and
conventional-DTI using box-and-whisker plot.
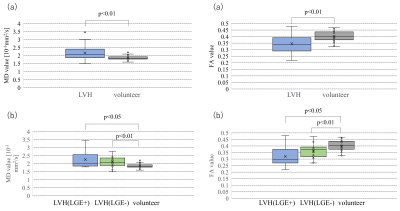
(a)The MD and FA values in LVH and
volunteer.
(b)The MD and FA values in LGE positive and
negative patients with HCM, and volunteer.
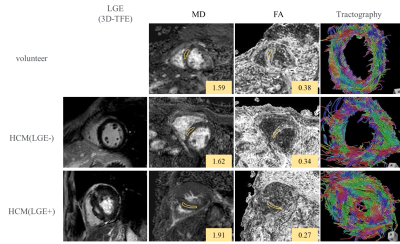
Representative cases of LVH with and
without LGE, and volunteer: Not only LGE positive but apparently LGE negative
segments also showed higher MD and lower FA values compared with volunteer.
Tractography showed the disarray of fiber architecture in LVH patients, even in
LGE negative case, compared with normal myocardium.
DOI: https://doi.org/10.58530/2022/4140