4138
High resolution Isotropic 3D Black Blood Thoracic Aorta Imaging With Low Rank Patch-Based Reconstruction1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, shenzhen, China, 2Shenzhen College of Advanced Technology, University of Chinese Academy of Sciences, shenzhen, China, 3Peking University Shenzhen Hospital, shenzhen, China
Synopsis
Three-dimensional (3D) black-blood MRI is a promising noninvasive imaging technique for assessing aortic atherosclerotic plaque. In this work, a low rank patch-based technique is proposed, and combined with a 3D modulated vFA-FSE sequence for high-resolution thoracic aorta imaging. The comparison was conducted on healthy volunteers and compared against a conventional GRAPPA acquisition to assess the feasibility of the proposed scheme. The results showed the reconstruction scheme was able to visualize the lumen areas clearly and improve the vessel sharpness and contrast ratio significantly.
Introduction
3D black-blood MRI is a promising noninvasive imaging technique for assessing aortic atherosclerotic plaque[1]. The high spatial resolution plays an important role in these plaque detection and identification. Because that the fibrous cap and lipid core are usually sub-millimeter in the physical dimension[2]. However, traditional MRI studies of the thoratic aorta wall had limited coverage, coarse through-plane resolution (1.3 mm-1.5 mm). More recently, 3D-variable flip-angle (vFA) SPACE (Sampling Perfection with Application-optimized Contrasts by using different flip angle Evolutions) had already been proven to be capable of assessing plaque burden in carotid, thoracic and abdominal aorta [3]. Here, we applied a 3D modulated vFA-FSE sequence and iterative low rank patch-based reconstruction to achieve an isotropic high resolution thoratic aorta wall imaging within 4.5 minutes. The studies primarily focused on the optimization of vFA-FSE sequence for the visualization of thoratic aorta wall with high resolution. This work was evaluated through in-vivo experiment and compared against the traditional GRAPPA to assess the feasibility of the proposed framework. Results have demonstrated that the visualization and sharpness of the vessel wall and the definition of the tissue boundary were in good agreement with the GRAPPA acquisition, which took approximately more than 7.2 min. Further valuation of this technique in patients will be conducted to determine its clinical use.Methods
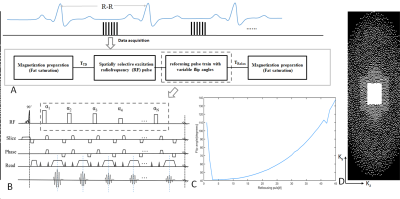
Sequence and Image Reconstruction: The schematic and timing diagram of the 3D T1-weighted black-blood modulated vFA-FSE sequence are shown in Figure 1. Specifically, a spatially selective radiofrequency (RF) excitation with an asymmetric pulse is applied for the selection, which can shorten the echo time and increase the vascular wall contrast. Specially, the short nonselective RF pulses are employed in the refocusing pulse train, which can shorten the echo spacing (ESP) and enhance the efficiency of data acquisition as compared to conventional slice-selective RF pulses (duration, several milliseconds). Figure 1C shows the flip angles with a refocusing pulse. Then, variable-density Cartesian sampling(Figure 1D) with compressed sensing (CS) is used to accelerate image acquisition and a iterative low rank patch-based algorithm for the imaging reconstruction[5] of sparsely undersampled k-space data.In the reconstruction, Let $$$X\in M^{N_{x}\times N_{y} \times N_{z}}$$$ be the image to be reconstructed, where $$$N_{x}$$$, $$$N_{y}$$$ and $$$N_{z}$$$ are the number of voxels in the $$$k_{x}$$$, $$$k_{y}$$$ and $$$k_{z}$$$ dimension. Due to the low SNR of the thoracic aorta imaging,the image can be decomposed into two parts based on the noise standard deviation map: one includes the bulk image signal,which can be denoted as $$$X{1}$$$ and the other incluces the noise, which can be denoted as $$$X{2}$$$. Then $$$X{1}$$$ can be expressed as a highorder low rank representation on a patch scale, with respect to an appropriately chosen patch selection operator $$$P_{p}(\cdot)$$$. The image reconstruction problem can be modeled as the following optimization on the high-order low-rank tensor $$$\tau$$$:
$$ {argmin_X \frac{1}{2}||EX-Y||_F^2+\sum_{p}\lambda_{p}||\tau_{p}||_{*} (1) \\ s.t. \tau_{p}=P_{p}(X_{1}), X_{1}=M_{1}X,X_{2}=M_{2}X } $$
Where $$$||\cdot||_{F}$$$ is the Frobenius norm; $$$E$$$ is the encoding operator[6]; $$$X$$$ is the image series to be reconstructed; $$$Y$$$ is the acquired k-space data; $$$||\cdot||_{\star}$$$ is the nuclear norm; $$$\lambda_{p}$$$ is a regularization parameter; $$$P_{p}(\cdot)$$$ forms a 3-way tensor from a patch centered at pixel p from a set of images[7]. M1 and M2 are the decomposition matrices based on the noise standard deviation map. By using the Lagrangian optimization scheme, Equation (1) can be solved through alternating direction method of multipliers (ADMM)[8].
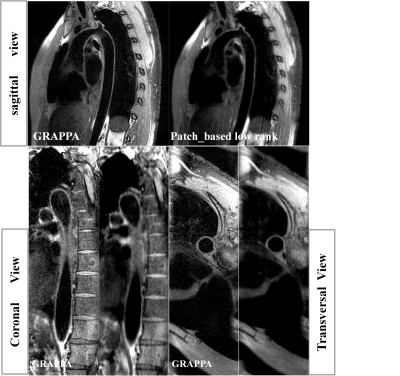
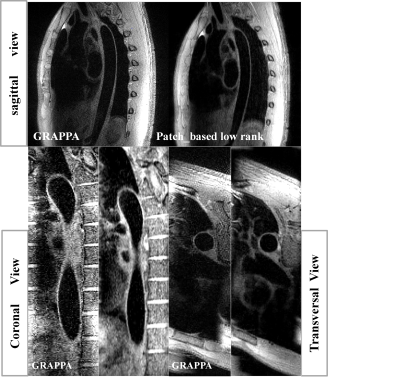
Experiments: All of the thoracic examinations were performed on a 3T whole-body MRI scanners during free-breathing (United Imaging, shanghai, China). This study was approved by our institutional review board and written informed consent was obtained before experiment. The 24-channel phased array abdomen coil was used for signal reception. For each scan, the modulated vFA-FSE sequence with variable-density sampling and traditional GRAPPA were acquired in the coronal plane during free breathing in the same subject. For the traditional GRAPPA, undersampled 2 in phase-encoding direction with elliptical k-space scanning (total acceleration of 3.06), ACS=32, Average=3.5, scan time>7.2 min. For the variable-density Cartesian sampling with accelerated factor of R=5.3 was executed, TE/TR=20.67/R-R ms, Average=3.5, scan time <4.3 min. Both of the two imaging schema were ECG-triggered to the mid-diastolic rest period (Trigger delay is about 500~700, depend on the cardiac cycle) and ETL=40~45, matrix=256 ×256×64, 1.0 isotropic resolution, BW=700Hz/pixel.
Results
The results show that the optimized vFA-FSE sequence combined with the patch based low rank reconstruction can achieve 3D thoracic aorta vessel wall imaging under free-breathing conditions within ~4.5 min and yield comparable thoracic aorta vessel visualization compared with the traditional GRAPPA approach. On average, It also reduces the acquisition time by 0.6 times compared with the traditional GRAPPA. The image results of Figure 2 and Figure 3 also demonstrate that the proposed patch based low rank reconstruction method can improve the vessel sharpness and the image contrast ratio.Conclusion
This study optimizes a high resolution, 3D MRI technique for thoracic aorta imaging using a vFA-FSE technique with iterative low rank patch-based reconstruction. The results demonstrate that the proposed scheme yields image quality comparable to that of traditional GRAPPA with longer acquisition time. Further evaluation of the proposed method in healthy volunteers and patients will be conducted in the future.Acknowledgements
This work was supported in part by the National Science Foundation of China (No.81901736, No.81571669, No.61471350, No.81729003, No. 61871373), Guangdong Provincial Key Laboratory of Medical Image Processing (No. 2017A050501026, No. 2018A0303130132). Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect those of the NSFC.References
[1] Z. Zhang, Z. Fan, T.J. Carroll, Y.C. Chung, P. Weale, R. Jerecic, D. Li, Three-dimensional T2-weighted MRI of the human femoral arterial vessel wall at 3.0 Tesla., Invest. Radiol. 44 (2009) 619–626. https://doi.org/10.1097/RLI.0b013e3181b4c218.
[2] M. Schär, W.Y. Kim, M. Stuber, P. Boesiger, W.J. Manning, R.M. Botnar, The impact of spatial resolution and respiratory motion on MR imaging of atherosclerotic plaque, J. Magn. Reson. Imaging. 17 (2003) 538–544. https://doi.org/10.1002/jmri.10287.
[3] G. Mihai, Y.C. Chung, A. Merchant, O.P. Simonetti, S. Rajagopalan, T1-weighted-space dark blood whole body magnetic resonance angiography (DB-WBMRA): Initial experience, J. Magn. Reson. Imaging. 31 (2010) 502–509. https://doi.org/10.1002/jmri.22049.
[4] M. Lustig, J.M. Pauly, SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space, Magn. Reson. Med. 64 (2010) 457–471. https://doi.org/10.1002/mrm.22428.
[5] A. Bustin, G. Lima da Cruz, O. Jaubert, K. Lopez, R.M. Botnar, C. Prieto, High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI, Magn. Reson. Med. 81 (2019) 3705–3719. https://doi.org/10.1002/mrm.27694.
Figures