4115
Characterizing the relative performance of a 13C/31P surface coil for simultaneously studying metabolism and energetics1Department of Radiology, UCSF, San Francisco, CA, United States, 2Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 3Center for Advanced Imaging Innovation and Research, and Center for Biomedical Imaging, Department of Radiology, New York Univerisity School of Medicine, New York City, NY, United States, 4Department of Hematology-Oncology, Beth Israel Deaconess Medical Center, Boston, MA, United States, 5Department of Radiology, Beth Israel Deaconess Medical Center, Boston, MA, United States
Synopsis
We evaluated the performance of a 13C/31P surface coil designed to detect metabolism and energetics data in the same scan session. Individually tuned coils were constructed to compare the performance of both channels of the multinuclear coil. Performance metrics such as Q ratio, SNR, flip angle maps, and transmit efficiency were measured. Our results demonstrate a decrease in performance of the 13C channel of the multinuclear coil. Removing the LCC trap circuit, used to decouple the channels, improved the Q ratio and SNR efficiency of the 13C channel. For future work, designs without a LCC trap circuit should be considered.
PURPOSE:
A multinuclear surface coil was built for studying metabolism from hyperpolarized carbon (13C) MR spectroscopy, and energetics from Phosphorus (31P) MRS in a single setting (1). To visualize low concentration metabolites such as bicarbonate, alanine, and endogenous phosphorus metabolites, high signal-to-noise ratio is critical. In this work, we evaluate the relative performance of the multinuclear coil as compared to single tuned surface coils by measuring transmit efficiency, SNR, flip angle maps, and Quality factor ratio. Assessing these performance metrics would allow for identifying potential design modifications to the existing multinuclear coil.METHODS:
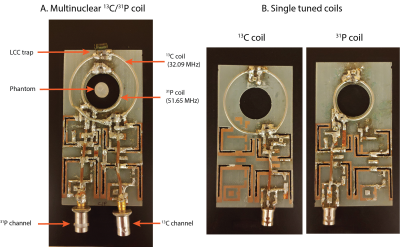
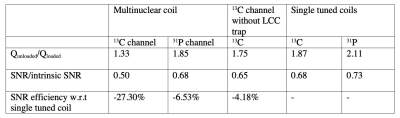
All experiments were carried out on a 3T animal MRI scanner (Biospec, Bruker, Billerica MA). The 13C/31P coil (Fig 1A) was constructed such that an inner loop with 3 cm diameter was tuned to 51.65 MHz (31P frequency) and a concentric outer loop with 5 cm diameter was tuned to 32.09 MHz (13C frequency). An LCC trap circuit (2) was included on the 13C channel to decouple the two channels (1). Separate single tuned coils with diameters matching the size of the 13C and 31P loops on the multinuclear coil were constructed (Fig 1B). Phantom localization was carried out using a volume proton (1H) coil (72 mm diameter, Bruker, MA).The Quality (Q) factor ratio (Qunloaded/Qloaded) was measured for all coils and for the case without LCC trap for the 13C channel (LCC trap replaced by effective capacitance). The SNR efficiency (SNRefficiency = Sqrt(1-Qloaded/Qunloaded)) was calculated from the Q values (3).
The optimal system reference power, i.e. transmit efficiency, was calculated using the small spherical phantom (6.65 M urea, 3.03 M diethyl(2-oxopropyl) phosphonate, doped with Gadolinium (Magnevist)) positioned at the center of the coil. The multinuclear coil was loaded with the saline phantom for all measurements. The input power required to obtain a 90 degree flip angle was calculated by varying the power for a series of single pulse acquisitions.
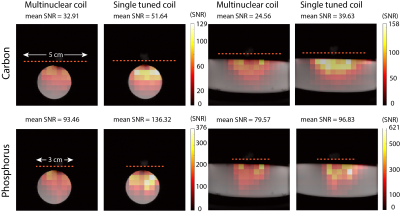
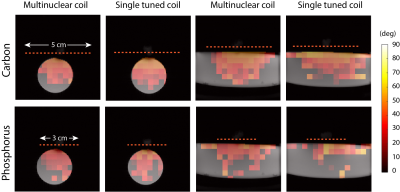
To characterize the performance of the coils spatially, SNR and flip angle maps were measured. The coils were loaded with a phantom consisting of 13C-urea and sodium phosphate (55mL: 0.92 M Sodium phosphate, 1.39 M 13C-Urea, 60 uL Gadolinium (Magnevist)). A small phantom filled with H2O was placed at the center of the coil for positioning the coil at the isocenter of the scanner. A 2D CSI sequence (flip angle = 90 degrees, slice thickness = 10 mm, matrix size = 16x16, FOV = 64 x 64, TR = 1s, averages = 2) was used to obtain spectroscopic images. The SNR was calculated voxel-by-voxel by dividing the peak absolute signal by the standard deviation of the real part of the first voxel, corresponding to a region outside of the imaging object. The same 2D CSI sequence was used for calculating flip angle maps using a double angle method (4) acquired with 90 and 45 degree flip angles. Thresholds were kept constant for each nucleus and applied to avoid calculating flip angle values in regions of low SNR. Based on the background proton images, masks were applied to both the SNR and flip angle maps. Data analysis was done in MATLAB (Mathworks, 2019).
RESULTS:
The carbon channel of the multinuclear coil required 81% higher transmit power, compared to the single tuned coil (Multinuclear coil 13C channel: 0.95 W, Single tuned 13C coil: 0.525 W), to achieve a 90 degree flip angle at the center of the coil. No difference in power was observed for the phosphorus channel (Multinuclear coil 31P channel: 0.07 W, Single tuned 31P coil: 0.07 W). The Q factor of the 13C channel of the multinuclear coil showed a decrease as compared to the single tuned coil with a reduction in SNR efficiency of 27.3%. On the other hand, the percent difference in SNR efficiency was 4.18% for the 13C channel without LCC trap, and 6.53% for the 31P channel of the multinuclear coil (Table). The SNR maps showed a reduced mean SNR for the multinuclear coil (Fig. 2) with a larger decrease in SNR for the carbon channel. Flip angle maps (Fig. 3) showed that the expected nominal flip angle of 45 degrees was achievable within the phantom when the system power was calibrated using the urea/diethyl(2-oxopropyl) phosphonate phantom.DISCUSSION and CONCLUSIONS:
This work evaluated the performance of a 13C/31P coil in comparison with individually tuned surface coils. A coplanar design of the multinuclear surface coil with a central aperture was originally designed for imaging the rodent brain (1). Our results demonstrated that the performance of the 13C channel of the multinuclear coil was impacted by the LCC trap circuit, which was included to decouple the two channels and improve coil sensitivity (2). Coil losses, introduced by the additional lumped elements on the LCC trap circuit, likely contributed to the lower transmit efficiency, Q ratio, and SNR of the carbon channel. As coil losses are dominant in coil SNR at low operating frequencies and for small imaging samples (5), modifications to the coil could include designs without the LCC trap circuit. For example, orthogonal coil elements that do not require a LCC trap circuit (6), or cryo-coils with low coil losses (7).Acknowledgements
This work was supported by NIH Training Grant T32CA151022, American Cancer Society Research Scholar Grant 18-005-01-CCE, NIH R01CA172845, NIH R01CA197254, NIH grant P41 EB013598 and P41 EB017183.References
1. Vaidya MV, Batsios G, Zhang Bei, et al. A 13C/31P surface coil to visualize metabolism and energetics in the brain. In: Proceedings of the 2020 International Society for Magnetic Resonance in Medicine Virtual Conference and Exhibition. 2020. Prog. no. 4110.
2. Meyerspeer M, Roig ES, Gruetter R, Magill AW. An improved trap design for decoupling multinuclear RF coils. Magnetic Resonance in Medicine 2014;72:584–590 doi: 10.1002/mrm.24931.
3. Rudin M. In-Vivo Magnetic Resonance Spectroscopy I: Probeheads and Radiofrequency Pulses Spectrum Analysis NMR, Basic Principles and Progress. Berlin, Heidelberg: Springer Berlin Heidelberg; 1992.
4. Stollberger R, Wach P, McKinnon G, Justich E, Ebner F. Rf-field mapping in vivo. In: Proceedings of the 7th Annual Meeting of SMRM, San Francisco, CA, USA 1988:106.
5. Doty FD, Entzminger G, Kulkarni J, Pamarthy K, Staab JP. Radio frequency coil technology for small-animal MRI. doi: 10.1002/nbm.1149.
6. Alfonsetti M, Sotgiu A, Alecci M. Design and testing of a 1.5 Tesla double-tuned (1H/ 31P) RF surface coil with intrinsic geometric isolation. Measurement: Journal of the International Measurement Confederation 2010 doi: 10.1016/j.measurement.2010.07.003.
7. Kovacs H, Moskau D, Spraul M. Cryogenically cooled probes—a leap in NMR technology. Progress in Nuclear Magnetic Resonance Spectroscopy 2005;46:131–155 doi: 10.1016/J.PNMRS.2005.03.001.
Figures