4112
B1+ efficiency of a single-loop dual-frequency surface coil for proton and deuterium or oxygen-17 magnetic resonance imaging at 16.4T1University of Minnesota, Minneapolis, MN, United States
Synopsis
We compared the B1 efficiency of a newly developed dual-frequency surface coil operating at proton and low-g X-nuclei frequencies at 16.4T with that of traditional proton coil and X-nuclei coil, respectively. Both simulation and experimental results revealed that the B1 field strength of the new coil was similar to that of traditional coil at deuterium or oxygen-17 frequency though was significantly reduced at proton frequency, while their B1 distributions were identical for both proton and deuterium/oxygen-17 frequencies. The efficacy of the new coil for in-vivo deuterium metabolic imaging applications was demonstrated in normal and tumor rat brains.
Introduction
X-nuclear (e.g., 31P, 17O and 2H) MRS imaging (MRSI) plays a critical role in understanding neuroenergetics and metabolism under various pathophysiological conditions1,2. Recently, we have developed a simple and cost-effective dual-frequency surface coil that can be tuned/matched to both proton frequency (~698 MHz) and low-g X-nuclei frequency (2H~107MHz or 17O~94.7MHz) for MRSI studies at 16.4T3,4. The simplicity and self-decoupling nature of this coil is attractive for assessing cerebral glucose and oxygen metabolism, blood flow, and TCA cycle activity using quantitative 2H and 17O MRSI technique at ultra-high field (UHF)1,5,6. To better understand the property and performance of this coil, RF simulation and experiments were carried out to investigate the RF coil efficiency of magnetic transmission field (B1) at both proton and X-nuclei frequencies, and compared with the traditional single-frequency coils. Using this novel dual-frequency coil, we also conducted deuterium metabolic imaging on in-vivo normal and tumor rat brains to demonstrate the excellent sensitivity efficacy of the new coil.Methods
B1 simulation:Three coil configurations with the corresponding LC resonant circuit as shown in Figure 1 were used for simulation. The simulation was conducted in CST studio suite 2020 (Dassault Systèmes, Vélizy-Villacoublay, France) and based on the hexahedral time-domain solver. In simulation, the water phantom and copper wire were constructed to match the experimental setup (water phantom, permittivity = 84, conductivity=0.112 S/m; copper wire, conductance = 5.96x107 S/m). For all models and boundary space around the models, we used 0.5 mm isotropic resolution mesh. The |B1+| maps are normalized by the forward Vrms seen by the coil.
B1 mapping:
The 1H B1 was mapped at 16.4T with a 3.5-cm diameter spherical water phantom based on the double-flip-angle method7 using the 2D gradient-echo (GE) multiple-slice (GEMS) imaging sequence (TR = 6 s, echo time (TE) = 2 ms, data matrix (DM) = 64 × 64, field of view (FOV) = 4.8×4.8 cm2, nominal pulse flip angle (FA) = 20° and 40°). While for X-nuclei (17O as an example), 3D chemical shift imaging (CSI) (TR=50ms, matrix=9×9×5, FOV=6×6×6cm3) sequences with FA = 40° and 80° were employed to acquire 17O images and generate 17O B1 maps.
In-vivo rat brain experiment:
One normal rat and one rat with brain tumor were scanned with the protocol approved by the Institutional Animal Care and Use Committee at University of Minnesota. Both animals were mechanically ventilated under 2% isoflurane anesthesia in a mixture of N2O/O2 (v/v=65:35) during the MRI/MRS measurements with stable physiological conditions. For the tumor rat brain study, D-Glucose-6,6-d2 was administrated intravenously via 2-min infusion. All MR experiments were conducted at 16.4 T/26 cm scanner (Varian/VNMRJ) using the 1H-2H dual frequency surface coil (2.2cm x 1.8cm). 2D GEMS sequence (TR = 165.9 ms, TE= 2.1 ms, FA = 25°, NT = 5, DM = 128 × 128, FOV = 4×4 cm2) was used to acquire proton MRI images. 3D CSI sequence (TR = 45 ms, DM = 9×9×5, FOV = 4×4×4 cm3, 69 s per CSI) was used to acquire 2H data. The 2H data were processed with LB=20Hz before Fourier transformation to enhance spectral signal-to-noise ratio. A Matlab-based program was used to fit all resonance signals.
Results
Figure 2(a) shows the simulated B1 field distribution at 1H frequency (first row) for the new dual-frequency coil (right) and the traditional coil (left) and the corresponding profile across the center of the phantom (second row). The B1 strength for the 1H traditional coil is about 2 times stronger than the dual-frequency coil, nevertheless the B1 field distributions for both coils are identical. In addition, current distributions on the coil wires are also shown for comparison (third row). Experimental results (Figure 2(b)) show consistent coil behaviors with the simulations at 1H frequency. In contrast, B1 field at 17O frequency is comparable between the two coils in both simulation (Figure 3(a)) and experiment (Figure 3 (b)). Overall results suggest that the new dual-frequency coil design provides optimal performance for low-frequency X-nuclei resonator and sub-optimal but acceptable performance for high-frequency 1H resonator (~7 times in frequency difference) in UHF imaging. In-vivo 2H MRSI for normal rat brain (Fig. 4a) and rat brain with tumor (Fig. 4b) show excellent sensitivity in capturing the metabolic changes in the brain and differentiation between normal and diseased brain tissues.Conclusion
In this work, we conducted thorough coil simulation and experiments to validate the excellent B1 efficiency of the newly developed dual-frequency surface coil at X-nuclei frequency and acceptable efficiency at proton frequency. In-vivo rat brain results show great potential for future applications.Acknowledgements
This work was supported in part by NIH grants of R01 MH111413, R01 CA240953,
U01 EB026978, S10 RR025031, P41 EB027061 and P30 NS076408.
References
1 Lu, M., Zhu, X.-H., Zhang, Y., Mateescu, G. & Chen, W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. Journal of Cerebral Blood Flow & Metabolism 37, 3518-3530 (2017).
2 Zhu, X.-H., Lu, M. & Chen, W. Quantitative imaging of brain energy metabolisms and neuroenergetics using in vivo X-nuclear 2H, 17O and 31P MRS at ultra-high field. Journal of Magnetic Resonance 292, 155-170 (2018).
3 Zhang GL et al. A dual-frequency surface coil design comprised of a single loop for both proton and deuterium magnetic resonance imaging at 16.4T. Proceedings of ISMRM 28: p. 4105 (2020).
4 Jenkins PJB et al. Single loop tri-frequency surface coil design for 1H MRI and interleaved dynamic 2H and 17O MRS applications at ultrahigh field of 16.4T. Proceedings of ISMRM 29: p. 1816 (2021).
5 Mateescu, G. D., Ye, A., Flask, C. A., Erokwu, B. & Duerk, J. L. in Oxygen Transport to Tissue XXXII. (eds Joseph C. LaManna et al.) 193-199 (Springer US).
6 Zhu, X.-H. & Chen, W. In vivo X-Nuclear MRS Imaging Methods for Quantitative Assessment of Neuroenergetic Biomarkers in Studying Brain Function and Aging. Frontiers in aging neuroscience 11: doi: 10.3390/metabo11030145 (2018).
7 Insko E, Bolinger L. Mapping of the radiofrequency field. Journal of Magnetic Resonance, Series A 103:82-85 (1993).
Figures
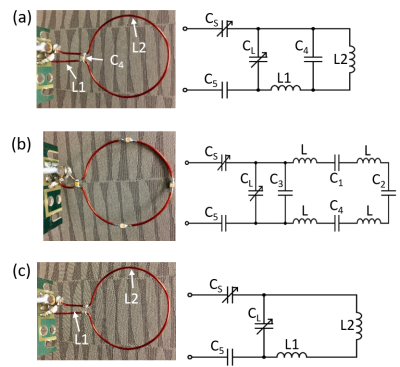
Figure 1. Newly designed dual-frequency single-loop surface coil (a) versus traditional 1H surface coil with 4 split capacitors (b) and low-g X-nuclei coil (c). RF coil circuit diagrams for each coil configuration are also shown.
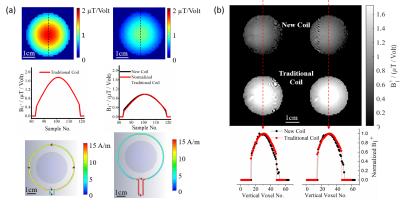
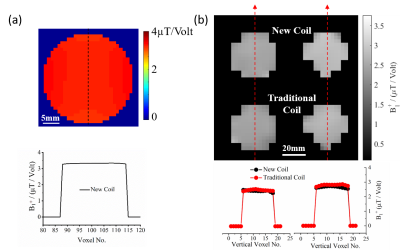
Figure 3 (a) Simulation result: B1 field distribution (top) and central B1 profile (bottom) for the dual-frequency coil operating at 17O frequency. (b) Experimental result: comparing B1 mapping (top), central B1 profile (bottom), between traditional single-frequency coil and the dual-frequency coil operating at 17O frequency as an example. Both simulation and experiment show the similar B1 distribution between the dual-frequency coil and traditional single-frequency coil.
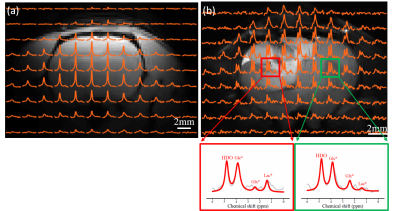
Figure 4. A 2H CSI image overlay on the corresponding proton image of a normal rat brain (a) and a rat with brain tumor (b). 2H CSI data in (b) were acquired around 25 min after D66 Glucose infusion. The representative deuterium spectra (original spectra in gray and fitted spectra in red) from two different voxels in the tumor rat brain are shown (tumor voxel in red and normal appealing voxel in green). The deuterium-labeled water (HDO) peak at 4.8 ppm, glucose (Glc*) at 3.87 ppm, glutamine/glutamate (Glx*) at 2.4 ppm, and lactate (Lac*) at 1.4 ppm can be easily identified.