4108
A High RF Bandwidth Diffusion Preparation for Diffusion Weighted Imaging Near Metallic Implants1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Bioengineering, Stanford University, Stanford, CA, United States
Synopsis
There is no widely adopted clinical protocol for diffusion weighted imaging near metal since the commonly used EPI trajectory fails completely due to distortion from off-resonance. We combine the magnetization prepared, stimulated echo diffusion preparation with 3D MSI encoding to enable volumetric diffusion weighted imaging near metal in clinically feasible times. Imaging time is reduced by a factor of 2× with high RF bandwidth root-flipped Shinnar-Le Roux refocusing pulses since spectral coverage can be maintained with fewer excitations. Joint design of the excitation and refocusing pulses reduces B1 sensitivity.
Introduction
Diffusion weighted (DW) imaging is a highly desired contrast for cancer diagnosis and treatment evaluation1. There is no widely adopted clinical protocol for DW imaging near metal since the commonly used EPI trajectory fails completely due to distortion from off-resonance (2-20$$$\,$$$kHz). Multispectral imaging (MSI) sequences such as MAVRIC-SL and SEMAC2,3 are highly effective at suppressing off-resonance artifacts from metal.A challenge in DW-MSI is that 3D encoding is required to resolve spins excited outside the slice, and Carr-Purcell-Meiboom-Gill (CPMG) spin-echo readouts are used to counteract T2*. The CPMG condition requires that the phase of the refocusing pulse be shifted 90° to the phase of the excitation4. If the CPMG condition is not met, the amplitude of the spin-echo is considerably reduced when the refocusing flip angle is not 180°. Non-CPMG magnetization resulting from diffusion gradients can be resolved using the stimulated echo diffusion preparation5 shown in Figure 1A.
In this work, we combine the magnetization prepared, stimulated echo diffusion preparation with SEMAC encoding. Imaging time is reduced by a factor of 2× with high RF bandwidth root-flipped Shinnar-Le Roux (SLR) refocusing pulses6 satisfying non-linear phase CPMG. High RF bandwidth allows spectral coverage to be maintained with fewer excitations.
Methods
In MSI, the excitation k-space velocity of the preparation and readout must match to ensure that the same spatial locations are prepared, excited, and imaged, regardless of resonance frequency. RF bandwidth should be maximized to excite spins adjacent to the implant, but short, high time-bandwidth (TBW) 180° pulses for diffusion preparation will exceed the peak B1 limit. However, this refocusing pulse need not have linear phase because signal attenuation from diffusion-sensitizing gradients is dependent on the magnitude of the nutation, not its axis of rotation. We can therefore employ SLR root-flipped pulses to obtain a non-linear phase refocusing with reduced peak B1.Non-linear phase from the root-flipped refocusing pulse can be eliminated using a twice-refocused preparation with linear phase excitation and tipup. However, this violates the CPMG condition at the slice level. Instead of linear phase 90s, identical phase pulses satisfying the beta profile:
$$\beta_{90}=i\beta_{180}/\sqrt2$$
can be used7. The CPMG condition is satisfied because the rotation axis of the refocusing pulse is offset 90° from the excitation everywhere within the non-linear phase slice (Figure 1B).
A minimum-phase SLR pulse (TBW=5, passband ripple $$$\delta_{1e}=0.005$$$, stopband ripple $$$\delta_{2e}=0.001$$$) was used to initialize the root-flipping algorithm.
The non-linear phase CPMG preparation was validated in phantom acquisitions at 3T (GE Healthcare Signa Premier).
B1 sensitivity was evaluated by acquiring a uniform agar ball using the following twice-refocused preparations: entirely linear phase pulses, root-flipped refocusing with linear phase excitation/tipup, and root-flipped refocusing with identical phase excitation/tipup.
The effect of B1 inhomogeneity and increased RF bandwidth was tested in a phantom consisting of a total shoulder replacement phantom with same preparations as above. Bin bandwidth: 600 Hz for linear phase refocusing, 1200 Hz for root-flipped refocusing, maximum B1 0.18G.
DW-MSI with identical phase excitation and root-flipped refocusing was appended to the clinical exam of a patient with a total hip arthroplasty at 1.5T (Optima 450W) following informed consent and IRB approval. FOV 45x36cm, matrix 256x100x32, 5 mm slices, 8 bins, bin bandwidth 1200 Hz, b-value=0,500s/mm2, duration 8:40. A low-resolution 2D projection of the phase was used to correct for motion-induced phase before shot combination.
Results
The refocusing pulse obtained using the root-flipping method and its identical phase pair are shown in Figure 2. Root-flipping reduced the peak B1 of the refocusing pulse from 0.34G to 0.19G for a 3.24 ms pulse with 1.6 kHz bandwidth.Comparison of preparations in the agar ball is shown in Figure 3. Employing root-flipped 180s with an identical phase excitation that satisfies the CPMG condition has similar shading to a solely linear phase preparation.
A fixed xy-slice in the shoulder phantom from excitations at different spectral offsets is shown in Figure 4. Root-flipped 180s permit the bin bandwidth to be doubled and excites spins closer to the implant. Signal recovery in these regions and at the edges of the phantom is improved using identical phase pulses.
Figure 5 shows application in vivo. Marked edema of the visualized extremity is due to treatment related lymphedema. Subcutaneous fluid visible in STIR MAVRIC-SL is visible in ADC maps obtained with DW-MSI. ADCs in the subcutaneous fluid pockets (dashed white arrows) are $$$2.5-3.0\times 10^{-3}$$$mm2/s but are partial volumed with fat. Soft tissue edema in the combined ROI indicated by dotted white arrows had an overestimated ADC of $$$3.5±0.3\times 10^{-3}$$$mm2/s.
Discussion
We have presented a sequence that combines a stimulated echo diffusion preparation with multispectral CPMG spin-echo trains, permitting diffusion weighted imaging with volumetric coverage near metal in clinically feasible scan times. Our method has increased spectral coverage and SNR efficiency compared to 2D DW-MSI8.Insufficient phase navigator resolution contributed to ADC overestimation. Application in vivo demonstrates that 3D DW-MSI suppresses distortion artifacts from metal and provides complementary contrast to conventional acquisitions.
Conclusion
Volumetric diffusion weighted imaging near metal can be achieved in clinically feasible scan times with a stimulated echo diffusion preparation and 3D CPMG spin-echo readouts. Root-flipped refocusing pulses increase spectral coverage and identical phase excitation reduces B1 sensitivity .Acknowledgements
GE Healthcare. R01 EB017739 . R01 AR077706.References
1. Padhani AR, Liu G, Mu-Koh D, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009;11(2):102–125.
2. Koch KM, Brau AC, Chen W, Gold GE, Hargreaves BA, Koff M, et al. Imaging Near Metal with a MAVRIC-SEMAC Hybrid. Magnetic Resonance in Medicine 2011 jan;65(1):71–82.
3. Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice encoding for metal artifact correction in MRI. Magnetic Resonance in Medicine 2009 jul;62(1):66–76.
4. Meiboom S, Gill D. Modified spin-echo method for measuring nuclear relaxation times. Review of Scientific Instruments 1958;29(8):688–691.
5. Zhang Q, Coolen BF, Versluis MJ, Strijkers GJ, Nederveen AJ. Diffusion-prepared stimulated-echo turbo spin echo (DPsti- TSE): An eddy current-insensitive sequence for three-dimensional high-resolution and undistorted diffusion-weighted imaging. NMR in Biomedicine 2017;30(7):e3719.
6. Pauly J, Nishimura D, Macovski A, Roux PL. Parameter Relations for the Shinnar-Le Roux Selective Excitation Pulse Design Algorithm. IEEE Transactions on Medical Imaging 1991;10(1):53–65.
7. Pauly JM, Wong EC. Non-Linear Phase RF Pulses for Reduced Dynamic Range in 3D Rare Imaging. In: Proceedings of the 9th Annual Meeting of ISMRM Glasgow, Scotland, UK; 2001. p. 688.
8. Koch KM, Bhave S, Gaddipati A, Hargreaves BA, Gui D, Peters R, et al. Multispectral diffusion-weighted imaging near metal implants. Magnetic Resonance in Medicine 2018;79(2):987–993.Figures
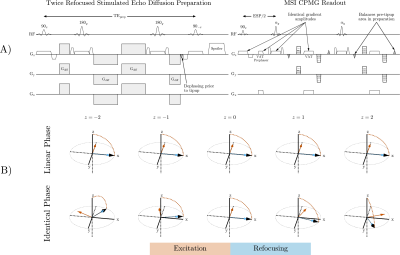
Figure 1: A) Stimulated echo preparation that corrects non-CPMG artifacts. View Angle Tilting (VAT) reduce in-plane distortion. B) Magnetization path for 90-180 pulse pairs. Magnetization rotates about the orange arrow during excitation and blue arrow during refocusing. Linear phase pulses have a rotation axis constant in the slice direction. The refocusing axis is offset 90° from the excitation to satisfy the CPMG condition. The identical phase pulse pair has non-linear phase but CPMG is maintained.
Figure 2: Top: Beta magnitude and phase of a minimum-phase, TBW 5 SLR pulse (A) compared to the beta profile of the minimum peak B1 RF obtained using root-flipping (B). The phase of the identical phase excitation beta profile (C) is equal to the refocusing pulse. Bottom: RF pulses obtained from beta profiles in the top row and a minimum phase alpha. Root flipping reduces peak B1 of the refocusing pulse from 0.34 G to 0.19 G.
Figure 3: Effect of excitation beta profile on B1 sensitivity of root-flipped refocusing pulses in a uniform agar ball. A) Center slice of the linear phase preparation with dotted yellow line showing location of 1D signal profile. B) A linear phase excitation with root-flipped 180 has evident B1 shading. C) An identical phase excitation with root-flipped 180 has similar shading to the linear phase preparation. D) B1 map. E) Signal profiles of B and C normalized to the linear phase preparation.
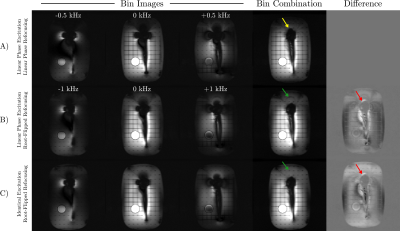
Figure 4: Bin images from different preparation schemes and the sum-of-squares bin combination. A) Preparation with only linear phase pulses reduces bin bandwidth, resulting in unexcited spins above the implant (yellow arrow). B) A root-flipped refocusing pulse enables greater bin bandwidth, improving signal recovery near the head of the implant (red arrows). C) An excitation with identical phase to the root-flipped refocusing pulse reduces B1 sensitivity above the implant (green).
Figure 5: Patient with unilateral hip replacement. Fluid that is bright on STIR MAVRIC-SL (dashed white arrows), appears on the ADC map as an elevated ADC. Soft tissue edema is indicated by the dotted white arrows. DW-MSI has reduced spectral coverage compared to MAVRIC-SL due to limited scan time, resulting in some signal appearing on MAVRIC-SL but not in DW-MSI (purple arrow).