4096
MR-guided Focused Ultrasound of the Dorsal Root Ganglion for the Treatment of Low Back Pain: Preclinical Study in a Peripheral Nerve Injury Model1University of Utah, Salt Lake City, UT, United States
Synopsis
Many of the currently available low back pain (LBP) treatments are invasive with associated risks and complications. MR-guided Focused Ultrasound (MRgFUS) is a lower risk, completely non-invasive alternative modality. We developed a large animal chronic LBP model, induced by peripheral nerve injury, and evaluated the response with quantitative sensory testing (QST). Here we investigate the ability to decrease neuropathic LBP with FUS neuromodulation of the spinal dorsal root ganglion with MRgFUS. Our data suggest that the QST techniques are sensitive indicators of pain and that effects on pain reduction can be detected using this clinically relevant evaluation method.
Introduction
Many of the currently available low back pain (LBP) treatments are invasive with associated risks and complications. Focused ultrasound (FUS) is a lower risk, completely non-invasive alternative modality. In vivo animal models have suggested dorsal root ganglia (DRGs) to play a role in pain propagation1 and neuropathic modulation. We developed a large animal chronic LBP model, induced by peripheral nerve injury (PNI), and evaluated the response to several stimuli with quantitative sensory testing (QST).2 Here we investigate the ability to decrease neuropathic LBP in the pig PNI model with FUS neuromodulation of the spinal DRG with MR-guided FUS (MRgFUS) without permanent surrounding tissue damage.Methods
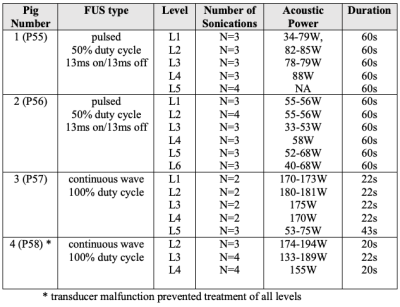
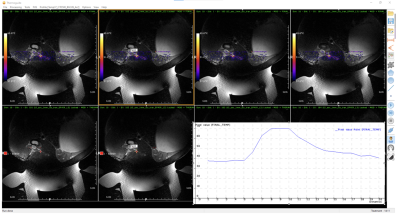
In an IACUC approved protocol, pigs (N=4) were trained to stand in a testing arena equipped with cameras and microphones and underwent weekly QST, using a porcine pain scale that uses vocalization and facial expression as quantifiable measures indicative of a supra-spinal pain response.2 This scale mimics techniques used in non-verbal humans who cannot self-report.3 QST was performed for 4 weeks before and 6 weeks after MRgFUS. Two animals served as controls, and two animals underwent surgery (unilateral ligation of the common peroneal nerve with complete Freund’s adjuvant- an inflammatory agent) to create neuropathic radicular LBP. For MRgFUS, the animals were positioned dorsal recumbent over the FUS transducer (IGT, f=1MHz, focal length = 10cm) and imaged with a custom built 3-channel coil at 3T (Siemens, PrismaFIT), as seen in Fig. 1. The transducer was positioned to unilaterally target the DRGs (L1 to L5/L6). Two different sonication protocols were used: continuous FUS sonication for the control animals and pulsed sonications for the PNI animals (details in Table 1). MR temperature imaging (MRTI) (3D segmented EPI, FOV=192x180x30 mm, Res=1.5x1.5x2.5 mm, TR/TE=23/11 ms, FA=14°, BW=1004 Hz/px, ETL=7) was used to measure temperature at the location of the DRG and surrounding areas. Post treatment T2w (3D SPACE to visualize edema) and CE-T1w (3D VIBE to visualize non-perfused volume) imaging was performed to assess damage to the area.Results
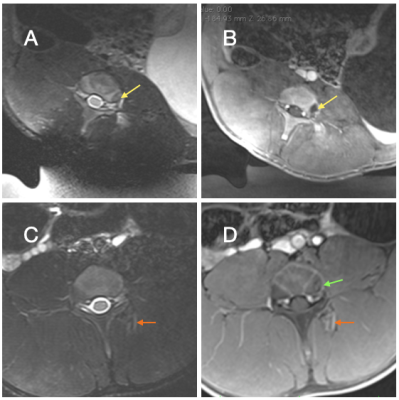
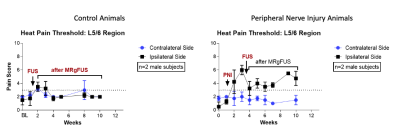
As per protocol, all animals received pain medication for three days after MRgFUS, but none of the animals showed pain or showed any motor deficits during standard veterinary assessments. The DRGs were easily localized, but direct targeting of the DRG was hindered by spinal bone (e.g. facet joint, transverse process). Moving the sonication focus lateral to the DRG allowed to target the spinal nerve. Temperature monitoring (Fig. 2) allowed visualization and temperature estimation in continuous sonications. In the longer duration pulsed sonications MRTI exhibited temperature drift and required correction in post-processing. Post treatment imaging in Figure 3 showed obvious edema in soft tissues and areas of nonenhancement and necrosis in animals undergoing continuous sonications, and mild edema in soft tissues and bone in animals with pulsed sonications. Of the different pain threshold tested, the heat pain threshold showed the largest change. Figure 4 left shows that in the control animals (high temperature ablation) pain scored to the heat pain threshold increased over baseline directly after MRgFUS and remained elevated for two weeks before returning to baseline. As seen in Figure 4 right, on the ipsilateral side (with injury), pain scores to the heat pain threshold QST test increased over baseline (weeks 0-1) after peripheral nerve injury (weeks 2-4) and then decreased for approximately 4 weeks after MRgFUS treatment to the lumbar DRG. There were no changes in the pain score to the heated thermode on the contralateral side.Discussion
The pig serves as a good animal model for human lumbar spine because of similarities in anatomy; however, we found that the larger pig posterior elements as compared to humans posed challenges targeting the lumbar DRG. This required adjustment of the focus slightly lateral to the neural foramen with ultrasound beam directed to the proximal spinal nerve. Temperature fluctuations caused by movement of gas filled bowel (mostly in sync with respiration, but irregular in some cases) corrupted several sonications, especially in the longer duration and pulsed sonications which required additional corrections performed in postprocessing.As pain is experienced at both the level of the spinal cord (reflexive) and the level of the brain (supraspinal), the lack of indicators of supraspinal sensation in most animal models of pain significantly reduces the clinical relevance of the evaluation. Therefore, we used QST outcome metrics that parallel highly quantitative clinical assessments of neuropathic pain. We were able to demonstrate expected increase in pain with PNI that was ameliorated for 4 weeks following MRgFUS treatment. Similar to Hellman et al4, we show that MRgFUS can ameliorate pain, and we further demonstrate that our QST techniques are sensitive indicators of pain in a large animal model and will be clinically translatable.
Conclusion
We demonstrated the ability to target the spinal DRG with MRgFUS without permanent surrounding tissue damage and showed a reduction in neuropathic low back pain in the pig. Our results suggest that the QST techniques are sensitive indicators of pain in a large animal model and that MRgFUS-effects on pain reduction can be detected using this clinically relevant evaluation method.Acknowledgements
NIH UH2 AR076736References
1) Gemes G, Koopmeiners A, Rigaud M, et al. Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C-type units after painful nerve injury. J Physiol. 2013;591(4):1111-1131.
2) Henley KY PB, Floyd CL. Development and Inter-rater Reliabiity of a Pig Evoked Pain Scale (PEPS) for use in a Porcine Model of SCI. Paper presented at: The 35th Annual National Neurotrauma Symposium2017; Snowbird, Utah.
3) Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231-243.
4) Hellman A, Maietta T, Clum A, Byraju K, Raviv N, Staudt MD, Jeannotte E, Ghoshal G, Shin D, Neubauer P, Williams E, Heffter T, Burdette C, Qian J, Nalwalk J, Pilitsis JG. Pilot study on the effects of low intensity focused ultrasound in a swine model of neuropathic pain. J Neurosurg. 2021 Apr 16:1-8. doi: 10.3171/2020.9.JNS202962. Epub ahead of print. PMID: 33862597.
Figures