4088
Quantum Sensing of Local Neuronal Firings (qsLNF) in Human Brain via Proton (1H) MRI: Proof of Concept
Yongxian Qian1, Liz Calderon1, Xingye Chen1, Anli Liu2, Yvonne W. Lui1, and Fernando E. Boada1
1Radiology, New York University, New York, NY, United States, 2Neurology, New York University, New York, NY, United States
1Radiology, New York University, New York, NY, United States, 2Neurology, New York University, New York, NY, United States
Synopsis
Neuronal firing generates fast action potentials along axon and slow postsynaptic currents at dendrites, which are difficult to detect and locate by scalp EEG and MEG placed above the skull. Here we propose local quantum sensing via endogenous proton (1H) nuclear spins inside firing neurons and detect magnetic fields indued by both action potentials and postsynaptic currents. Computer simulations and human studies showed that the proposed technique has the potential to non-invasively detect and locate neuronal firings in the brain through the acquisitions of FID signal on a 3T MRI scanner with multi-channel array coil.
INTRODUCTION
Neuronal firing generates an action potential traveling down axon and across synapses for communication among neurons. Traveling action potentials generate electric current in the axon (~2ms in duration) and in the postsynaptic dendrites (~10–100ms in duration). These currents produce electric and magnetic fields detectable by scalp electroencephalography (EEG) and magnetoencephalography (MEG), respectively1,2. However, scalp EEG and MEG detect the fields relatively far away (~20mm) from firing sources, and have difficulty to locate firing sources and thus limit their sensitivity to slow, easily-synchronized postsynaptic currents in a large area1,3-5. Here we propose to use local quantum sensing 6 and detect magnetic fields related to both fast (action potential) and slow (postsynaptic) neuronal currents with high temporal resolution and high spatial localization of firing sources.THEORY
The quantum sensors in our method are local endogenous nuclear spins of proton (1H) inside neurons, with two quantum states, |0> and |1>, in a static external magnetic field B0 provided by MRI scanner. Target magnetic field, Bn(r,t), to be measured at location r and time t in the brain is induced by electric currents, {In(r,t), r in ΔV}, generated by firing neurons nearby (<1mm in distance) 7-9. The sensing process is electromagnetic interaction of these nuclear spins at superposition state (achieved by an exciting RF pulse) with the target magnetic field (Fig. 1). During sensing period, nuclear spins accumulate an extra phase φ(r,t) (Eq. 1) and encode it in the free induction decay (FID), s(t), in Eq. 2. Spins' states are read-out by RF receive coils placed around the head during data acquisition of the FID signal from the spin ensemble in a coil-specific investigation volume, ΔV, of magnetization density ρ(r) and effective transverse relaxation constant T2*(r) (Eq. 2). A phase difference at a sampling interval Δt is calculated for an estimate of the firing field z-component Bn,z (Eq. 3). The firing magnetic field, although very small (~0.1 nT) above the scalp1,7-9, is large enough (~18–363 µT) inside axons (~0.2–2.0 µm in diameter) in the brain to generate a measurable change in local resonance frequency as large as 15.5 kHz for proton (1H) MRI.Eq. [1]. $$$ φ(r,t)=φ(r,TE)+γ∫_{TE}^t B_{n,z} (r,τ) dτ $$$.
Eq. [2]. $$$ s(t)=∫_{∆V} c(r) ρ(r) e^{-t/T_2^* (r)} e^{-jφ(r,t)} dr $$$
Eq. [3]. $$$ B ̃_{n,z} (t)=(1/γ)dφ/dt≈(1/γ) phase[s(t) s^* (t+∆t)]/(∆t) $$$, s.t. spatially uniform Bn,z across ∆V.
METHOD
Computer simulations and human studies were performed to determine the accuracy of qsLNF measurements and to show its feasibility in vivo. There were 40 FID signals acquired on four healthy subjects (age 28.0±5.4 years, 3 male and 1 female). All study subjects were provided the informed consent for the study. The FID signals were acquired at resting state using vendor’s pulse sequence AdjFre on a 3T scanner (Prisma, Siemens), with a standard head/neck array coil (16 out of 20 channels were used). The investigation volumes in the brain were defined by individual coil images (Fig. 2). The FID readout time is 100ms at a sampling interval of 0.0977ms, with TE=15ms and flip angle=10º. A custom-developed software in MATLAB R2021a (MathWorks, Natick, MA) was used to analyze the raw data, including a 3-point smoothing before and after the phase calculation in Eq. 3.RESULTS
The computer simulations in Fig. 3 showed a very high accuracy of the qsLNF measurements, with an error of <0.7% for the action potentials and <1.9% for the postsynaptic currents under a typical SNR=250. The human study in Fig. 4 presented a quiet resting state, only recording one postsynaptic firing (20ms long, 0.26µT intensity). In contrast, the subject in Fig. 5 showed an active rest state, with massive action potential firings (1.5–2.0ms long, 20–40µT intensity) from the prefrontal lobes and occipital lobes.DISCUSSION
The results in Figs. 2–5 showed the feasibility of our method that was based on quantum sensing of local neuronal firings. Proton (1H) nuclear spins, serving as intrinsic micro quantum sensors, exist inside neuons and thus have higher sensitivity to neuronal firings than scalp EEG and MEG above the skull. However, the estimates of neuronal magnetic field in Eq. 3 is prone to partial volume effect and needs to be further addressed. The sensing volume, passively defined by coil sensitivity in this study, needs to be reduced in size or actively defined by single-voxel excitation. The dynamic range of quantum sensing for magnetic field was restricted by sampling interval, ∆t, as seen in the design of simulation model for action potentials in Fig. 3a. To increase the dynamic range, ∆t needs to be decreased.CONCLUSION
The presented data of computer simulations and human studies supported our proposed idea for the quantum sensing of local neuronal firing via proton (1H) MRI. The qsLNF has the potential to non-invasively record neuronal firings of both fast action potentials and slow postsynaptic currents in the brain through continuous acquisition of FID signals. In principle, this idea can be extended to the deep brain and other body parts.Acknowledgements
This work was financially supported in part by the NIH RF1 AG067502 and the General Research Fund of the Department of Radiology. This work was also performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), an NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
1. Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407-420.2. Hennig J, Kiviniemi V, Riemenschneider B, et al. 15 Years MR-encephalography. MAGMA. 2021; 34:85-108.
3. Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Applied Magnetic Resonance. 2005; 29:65–88.
4. Boudurka J, Bandettini PA. Toward direct mapping of neuronal activity: MRI detection of ultra-weak, transient magnetic field changes. Magn Reson Med. 2002;47(6):1052-1058.
5. Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nat Neurosci. 2017; 20:327–339
6. Degen CL, Reinhard F, Cappellaro P. Quantum sensing. Reviews of modern physics. 2017 Jul 25; 89(3):035002.
7. Wikswo JP, Barach JP, Freeman J. Magnetic field of a nerve impulse: first measurements. Science. 1980 Apr 4;208(4439):53-5.
8. Swinney KR, Wikswo Jr JP. A calculation of the magnetic field of a nerve action potential. Biophysical journal. 1980 Nov 1;32(2):719-31.
9. Scott AC. The electrophysics of a nerve fiber. Reviews of Modern Physics. 1975 Apr 1;47(2):487.
Figures
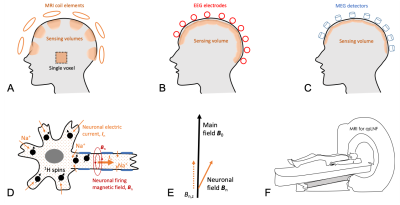
Fig. 1. Conceptual schematic of the quantum sensing of local neuronal firings. a) MRI-based qsLNF and sensing volumes defined by individual coil sensitivity (shading areas) or by selective single-voxel excitation (dashed line), compared with those of b) scalp EEG and c) scalp MEG. d) Micro quantum sensors of endogenous proton (1H) nuclear spins (black dots with arrows) immersed in sodium (Na+) ionic flow (neuronal current) inside a firing neuron. e) Quantum sensing of neuronal magnetic field Bn via the perturbation in MRI main field B0. f) Recording of neuronal firing via MRI scanner.
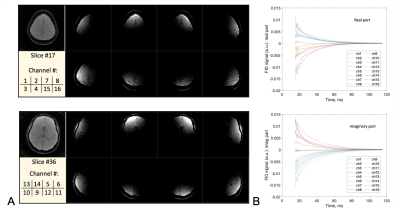
Fig. 2. MRI images and FID recordings from the brain of a study subject acquired by the qsLNF with a 16-channel receive coil array, showing the definitive source localization of qsLNF recordings. a) MRI images of sensing volumes defined by a subgroup of 8 coil elements at the top (slice #17) and middle (slice #36) levels of the brain. b) FID signals (real and imaginary) from all the 16 coil elements, with SNR = 675 (Ch8) – 2551 (Ch1). These images and signals demonstrate a definitive connection between the qsLNF measurements and their locations of signal sources.
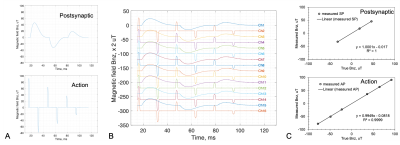
Fig. 3. Computer simulations of the qsLNF recording. a) The models of neuronal magnetic field Bn,z induced by postsynaptic currents (40ms-long for each firing) and action potentials (2ms-long for each firing). b) The qsLNF recordings transformed from the FID signals (Δt=0.0977ms, SNR=250, Gaussian noise independent across channels). c) Quantitative comparison of the peaks between the qsLNF measurements in b) and true values in a). The error bars in c) are too small to be visible. The relative error of the qsLNF measurements is <0.7% (action) and <1.9% (postsynaptic).

Fig.
4. A healthy
subject study
(25
years old, female). a) The qsLNF recordings at resting state and
associated sensing regions (MRI images attached to each channel) from the brain. b)
The FID signals (real and imaginary) that
were transformed into the qsLNF recordings in a). A postsynaptic signal
(~20ms in duration, dashed circle) is visible at Channel #14 (left-side prefrontal lobe). The
waves at the beginning period of time (15–40ms) in a) and b) are artifacts from
fat signal.

Fig.
5. Another healthy
subject study
(25
years old, male). a) The resting-state qsLNF
recordings and associated sensing regions from the brain. b)
The FID signals corresponding to the qsLNF
recordings in a). Massive firings of action potential (~2ms in duration) are
clearly visible at multiple channels including #7 and #14 (prefrontal lobes)
and #15 and #16 (occipital lobes).
DOI: https://doi.org/10.58530/2022/4088