4087
Disposable, Handheld Clinical-Scale HP Propane Hyperpolarizer for Pulmonary MRI1Chemistry and Oncology, Wayne State University, Detroit, MI, United States, 2Chemistry, Wayne State University, Detroit, MI, United States, 3International Tomography Center RAS, Novosibirsk, Russian Federation, 4Boreskov Institute of Catalysis RAS, Novosibirsk, Russian Federation, 5Radiology, Michigan State University, Lansing, MI, United States, 6Southern Illinois University Carbondale, Carbondale, IL, United States
Synopsis
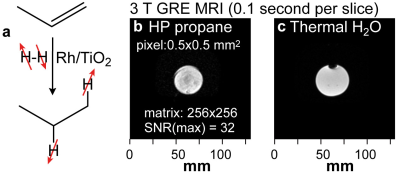
We demonstrate a disposable and handheld hyperpolarizer for clinical-scale production of hyperpolarized propane gas for application as an inhalable contrast agent for pulmonary MRI. The device consists of an aluminum commercial spray can, which contains a mixture of pressurized propylene and parahydrogen gases. This filled container can be easily transported and stored for weeks. The hyperpolarized propane production is initialized by valve actuation, which releases the propylene-parahydrogen mixture into a mini-catalytic reactor producing a stream of pure (without catalyst) hyperpolarized propane gas on demand. The feasibility of 3 T EPI, FIESTA, and GRE MRI in hyperpolarized phantoms is demonstrated.
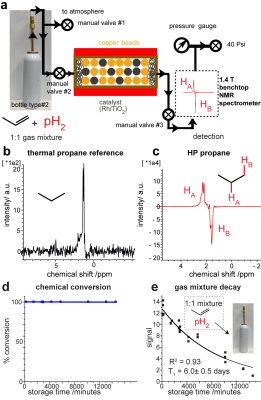
METHODS: We employ a 250 mL commercially available aluminum spray can for temporary storage of pressurized mixture of propylene:parahydrogen (1:1 and 1:4) gases at ~9 atm total pressure. We have employed 98.5% parahydrogen prepared using ultra-high purity hydrogen gas4. The disposable storage container is equipped with high-flow valve (>0.5 standard liters per second flow). The storage container shown in Figure 2 is mated to the disposable reactor made of food-grade copper tubing filled with 100 mg of 1% Rh/TiO2 catalyst and copper beads. The beads are added to improve thermal management2. Once the valve is actuated, the compressed gas mixture is released through the mini-reactor resulting in production of hyperpolarized propane gas stream. The performance of the hyperpolarizer was monitored using 1.4 T bench-top NMR spectrometer running dynamic pseudo-2D NMR, Figures 2 and 3. Custom MATLAB code3 was employed for all spectroscopic data processing and determination of relaxation rates and signal enhancement values. All reported imaging studies were performed using a 3.0 T MRI scanner and 2D EPI, GRE and FIESTA pulse sequences. The acquisition parameters are provided in corresponding Figure captions. The photograph of the experimental setup employed for imaging studies is shown in Figure 4.
RESULTS AND DISCUSSION: Parahydrogen gas1 serves as a source of hyperpolarization (Figure 1) in parahydrogen induced polarization of propane gas via pairwise addition of parahydrogen. The utility of a a commercial aluminum spray can as a temporary storage vessel was evaluated by monitoring the decay of the gas mixture potency over time: parahydrogen decays with the exponential rate constant of 6.0+/-0.5 days, Figure 2e. We conclude that this decay rate is sufficient for temporary storage of the gas mixture and overnight shipping.
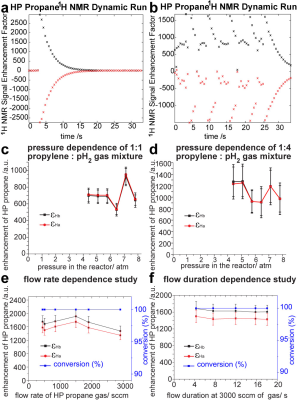
Systematic studies of flow rate, flow duration, and reaction pressure revealed that the production of HP propane gas is very robust (Figures 2 and 3) leading to apparent polarization values of 1-1.5% as detected at 1.4 T—in line with our previous studies2,5 using a benchtop polarizer. Although the polarization levels are substantially lower than those typically obtained for HP 129Xe, we note that each HP molecule carries double the polarization load (two HP protons versus one HP 129Xe atom), and each proton is typically 3.6 times more sensitive than 129Xe at the same nominal polarization. Moreover, protons have nearly 100% natural abundance versus 26% natural abundance of 129Xe. These compounding factors make 1.5%-hyperpolarized propane equivalent to 43%-hyperpolarized 129Xe – this math does not even factor in higher detection frequency of protons and highly-optimized RF coils already available in the clinical practice, which additionally favor HP propane imaging.
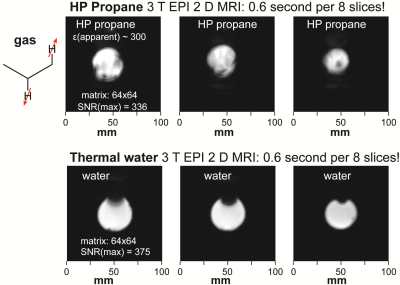
For 3T imaging studies, we employed a simplified setup without reactor heating as shown in Figure 4. We note that the valves are introduced only for the purpose of the phantom studies and will be obviated for future in vivo studies. Feasibility, of 2D GRE imaging is shown in Figure 1. Feasibility of multi-slice sub-second 2D EPI is demonstrated in Figure 5 --- three selected slices are shown from the stack of 8 images. Multiple 2D images were obtained from the same batch of HP propane gas, allowing one to monitor gas injection in the phantom and the hyperpolarization decay. We note that this technology will additionally benefit from low-field MRI5,6, which allows protecting the long-lived spin states of HP propane gas--therefore allowing for a substantially longer time window of gas preparation, administration, and imaging.
CONCLUSION: We conclude that a parahydrogen:propylene mixture can be successfully stored for days in a disposable handheld container that can be potentially shipped. Robust performance of the disposable handheld hyperpolarizer was demonstrated in vitro. The feasibility of ultra-fast 2D EPI, GRE, and FIESTA imaging of HP propane has been demonstrated. This pilot study bodes well for near-future in vivo validation of this prospective inhalable contrast agent for functional lung imaging applications. All-in-all, the new instrumentation presented here enabled low-cost on-demand production of HP propane using a handheld device, and imaging using conventional clinical MRI scanners.
Acknowledgements
This work was supported by DOD CDMRP W81XWH-15-1-0271 and W81XWH-15-1-0272, W81XWH-20-10576 and W81XWH-20-10578, NSF CHE-1904780 and CHE-1905341, NHLBI R21HL154032 and F32HL160108. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Bowers CR, Weitekamp DP. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical-Reaction and Nuclear-Magnetic-Resonance. Phys Rev Lett. 1986;57(21):2645-2648.
2. Salnikov OG, Nikolaou P, Ariyasingha NM, Kovtunov KV, Koptyug IV, Chekmenev EY. Clinical-Scale Batch-Mode Production of Hyperpolarized Propane Gas for MRI. Anal Chem. 2019;91(7):4741–4746.
3. Birchall JR, Irwin RK, Chowdhury MRH, Nikolaou P, Goodson BM, Barlow MJ, Shcherbakov A, Chekmenev EY. Automated Low-Cost In Situ IR and NMR Spectroscopy Characterization of Clinical-Scale 129Xe Spin-Exchange Optical Pumping. Anal Chem. 2021;93(8):3883-3888.
4. Nantogma S, Joalland B, Wilkens K, Chekmenev EY. Clinical-Scale Production of Nearly Pure (>98.5%) Parahydrogen and Quantification by Benchtop NMR Spectroscopy. Anal Chem. 2021;93(7):3594–3601.
5. Ariyasingha NM, Salnikov OG, Kovtunov KV, Kovtunova LM, Bukhtiyarov VI, Goodson BM, Rosen MS, Koptyug IV, Gelovani JG, Chekmenev EY. Relaxation Dynamics of Nuclear Long-Lived Spin States in Propane and Propane-d6 Hyperpolarized by Parahydrogen. J Phys Chem C. 2019;18(123):11734–11744.
6. Kovtunov KV, Truong ML, Barskiy DA, Koptyug IV, Coffey AM, Waddell KW, Chekmenev EY. Long-lived Spin States for Low-field Hyperpolarized Gas MRI. Chem Eur J. 2014;20(45):14629–14632.
Figures