4085
Simultaneous Time-of-Flight (TOF) MR Angiography, SWI and QSM without compromising TOF features1Biomedical Engineering, University of Alberta, Edmonton, AB, Canada
Synopsis
Multiple-echo gradient echo sequences developed to simultaneously compute TOF-MRA, SWI and QSM in previous studies have either compromised TOF-MRA features or have not computed QSM. In this abstract, images were acquired in healthy volunteers at 3T with different parameter variations of the proposed 3D triple-echo gradient echo sequence with all TOF-MRA features. The effect of TOF-MRA features on phase and QSM were explored and corrected to obtain artifact-free QSM. The parameters of the sequence have been optimized to obtain TOF-MRA, SWI, QSM and R2* maps simultaneously.
Introduction
Susceptibility Weighted Imaging(SWI) and Quantitative Susceptibility Mapping(QSM) offer means to study veins, lesions, hematoma etc1-4. QSM can be computed from the same sequence as SWI. Time-of-flight MR angiography(TOF-MRA) is clinically used to examine the arterial lumen5,6. Previous studies have applied multiple-echo gradient echo sequences to achieve both TOF and SWI/QSM from the same sequence7-12. However, these papers either did not compute QSM or have compromised the TOF-MRA acquisition by not including all the standard TOF-MRA features. Hence, the effect of the TOF-MRA parameters on phase and QSM is yet to be fully explored.Aim
To establish a TOF-QSM sequence for the brain with all features of standard TOF-MRA, including venous saturation pulse, ramped RF and multiple overlapping thin slab acquisition (MOTSA), and explore the effects of these parameters on QSM and SWI. Thus, attempting to optimize sequence parameters for TOF-MRA, SWI and QSM simultaneously.Method
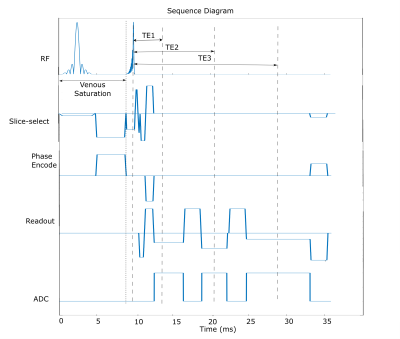
Sequence design:Fig 1 demonstrates the triple-echo 3D gradient echo sequence with ramped RF excitation and venous saturation for TOF-QSM. To minimize echo time for TOF imaging, the first echo is asymmetric, and the sequence is flow compensated in readout and slice-select direction. A middle echo is added to allow a first order gradient nulling in the readout direction for the third echo. MOTSA was also implemented in the introduced triple echo sequence.
Data acquisition:
Images were acquired at 3T in a uniform phantom and two healthy volunteers with the triple-echo TOF-QSM sequence(Fig 1) having parameters as follows:
(1) High resolution: 0.26×0.26×0.75mm3 with interpolation(for MRA); 0.52×0.52×0.75mm3 without interpolation(for SWI and QSM computation); acquisition time:8min 43s.
(2) Lower resolution: 0.38X0.38X0.80mm3 with interpolation(for MRA); 0.76X0.76X0.80mm3 without interpolation(for SWI and QSM computation); acquisition time:5min 33s.
Both sequences had TE1/TE2/TE3/TR=3.9/10.6/19/36ms; ramped profile RF excitation with FA=18° (14.8°-21.2°); 5 slabs(30 slices each) with 20% overlapping for MOTSA; venous saturation pulse before RF excitation and GRAPPA acceleration factor of 2.
The standard single-echo TOF sequence for comparison had: TE/TR=3.4/21ms; resolution: 0.26×0.26×0.75mm3; FA=18° (14.8° to 21.2°); 5 slabs(30 slices each) with 20% overlapping for MOTSA; venous saturation pulse before RF excitation; acquisition time of 5min 21s with a GRAPPA acceleration factor of 2.
Image Reconstruction:
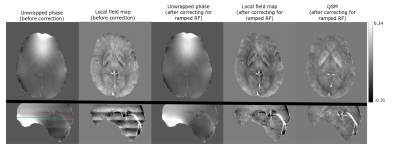
The overlapped MOTSA slabs were concatenated by removing three slices in the overlap region of each slab. SWI and QSM were reconstructed from the third echo. SWI images were generated using the standard method13,14. The ramped RF creates an inherent phase variation across the slab which can be observed in the raw phase used for QSM reconstruction. To correct for this, phase variation across the slab at the centre of the FOV was computed by subtracting the unwrapped phases with and without ramped RF pulse for a uniform phantom. This phase variation was then filtered to produce a correction factor which was applied to all the phase datasets. QSM computation steps included phase unwrapping with Prelude15, phase correction to remove the ramp RF phase variation and background removal with LBV16 followed by dipole inversion.
Results
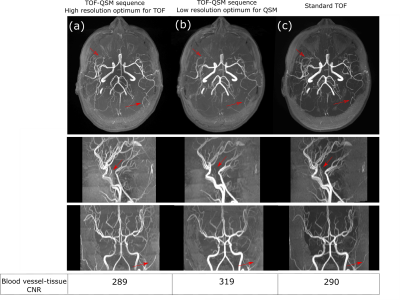
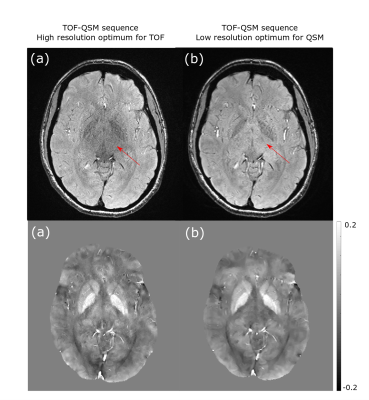
Fig 2 shows the effect of ramped RF on QSM reconstruction, by demonstrating variation on phase and local field, and then corresponding images after correction. Fig 3 and Fig 4 show the effect of SNR and resolution on MRA from the first echo and SWI, QSM from the third echo. Fig 3 lists the artery to background CNR on the magnitude images from the first echo. For the lower resolution acquisition, the QSM quality improved due to higher SNR, but the TOF-MRA was unable to depict some of the smaller arteries(Fig 3b,4b). The MRA computed from the sequence with optimized resolution of TOF had similar CNR as standard TOF depicting the smaller arteries and the QSM from the third echo, though noisier than low resolution one, was of acceptable quality (Fig 3a,4a).Discussion
A modified triple echo sequence was introduced to allow simultaneous computation of TOF-MRA, SWI and QSM while maintaining key TOF features of ramped RF excitation, venous saturation and MOTSA acquisition. Since it has 3 echoes, R2* maps can also be computed . It was observed that even after the venous saturation pulse, there was still enough signal in the veins at the third echo to compute QSM. Typically TOF-MRA coverage is less than the whole brain which is often used in QSM. Here a whole brain FOV was used.The ramped RF excitation provides a better depiction of arteries in MRA but causes a phase variation across the slab which is compensated by the phase filtering in SWI. However, this phase variation gives rise to artifacts in QSM which utilizes raw phase. Artifact-free local field and susceptibility maps were computed after correcting for the phase variation.
Previous work has shown that MOTSA, which is beneficial for artery depiction in TOF-MRA, reduces tissue SNR in proportion to √Nslab 9,10. TOF-MRA also requires high resolution which further reduces SNR which is compounded by using a larger flip angle above the background Ernst angle. However, QSM requires high phase SNR and hence the TOF matrix size was reduced while computing SWI and QSM for all acquisitions.
Conclusion
The proposed sequence allows simultaneous TOF-MRA, SWI, QSM and R2* map computation of the whole brain without compromising the TOF features.Acknowledgements
Grant support was provided by the Canadian Institutes of Health Research (CIHR). We thank Peter Seres for assistance with data collection.
References
1. Liu S, Buch S, Chen Y, Choi HS, Dai Y, Habib C, Hu J, Jung JY, Luo Y, Utriainen D, Wang M, Wu D, Xia S, Haacke EM. Susceptibility-weighted imaging: current status and future directions. NMR Biomed. 2017;30(4):10.
2. Wang S, Lou M, Liu T, Cui D, Chen X, Wang Y. Hematoma volume measurement in gradient echo MRI using quantitative susceptibility mapping. Stroke. 2013;44(8):2315-7.
3. Halefoglu AM, Yousem DM. Susceptibility weighted imaging: Clinical applications and future directions. World J Radiol. 2018;10(4):30-45.
4. Liu C, Wei H, Gong NJ, Cronin M, Dibb R, Decker K. Quantitative Susceptibility Mapping: Contrast Mechanisms and Clinical Applications. Tomography. 2015;1(1):3-17.
5. Graves MJ. Magnetic resonance angiography. Br J Radiol. 1997; 70:6-28.
6. Wehrli FW, Shimakawa A, Gullberg GT, MacFall JR. Time-of-flight MR flow imaging: selective saturation recovery with gradient refocusing. Radiology. 1986;160(3):781-5.
7. Boeckh-Behrens T, Lutz J, Lummel N, Burke M, Wesemann T, Schöpf V, Brückmann H, Linn J. Susceptibility-weighted angiography (SWAN) of cerebral veins and arteries compared to TOF-MRA. Eur J Radiol. 2012;81(6):1238-45.
8. Chen L, Zhang J, Wang QX, Peng L, Luo X, Zhu WZ, Roshan AK, Qi JP, Wang H. Enhanced susceptibility-weighted angiography (ESWAN) of cerebral arteries and veins at 1.5 Tesla. Br J Radiol. 2014;87(1039):20130486.
9. Deistung A, Dittrich E, Sedlacik J, Rauscher A, Reichenbach JR. ToF-SWI: simultaneous time of flight and fully flow compensated susceptibility weighted imaging. J Magn Reson Imaging. 2009 Jun;29(6):1478-84.
10. Do WJ, Choi SH, Park SH. Simultaneous Variable-Slab Dual-Echo TOF MR Angiography and Susceptibility-Weighted Imaging. IEEE Trans Med Imaging. 2018;37(7):1632-40.
11. Park SH, Shim H, Moon CH, Kim JH, Bae KT. Quantitative evaluation of k-space reordering schemes for compatible dual-echo arteriovenography (CODEA). Magn Reson Med. 2010;63(5):1404-10.
12. Chen Y, Liu S, Buch S, Hu J, Kang Y, Haacke EM. An interleaved sequence for simultaneous magnetic resonance angiography (MRA), susceptibility weighted imaging (SWI) and quantitative susceptibility mapping (QSM). Magn Reson Imaging. 2018;47:1-6.
13. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004;52(3):612-8.
14. Rauscher A, Barth M, Herrmann KH, Witoszynskyj S, Deistung A, Reichenbach JR. Improved elimination of phase effects from background field inhomogeneities for susceptibility weighted imaging at high magnetic field strengths. Magn Reson Imaging. 2008;26(8):1145-51.
15. Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–97.
16. Zhou D, Liu T, Spincemaille P, Wang Y. Background field removal by solving the Laplacian boundary value problem. NMR Biomed 2014;27: 312–19.
Figures