4084
Dynamic 3D Stack-of-Radial PRF MR Thermometry to Monitor High-Intensity Focused Ultrasound Heating: Validation in a Tissue Motion Phantom1Department of Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Department of Radiology, Mayo Clinics, Rochester, MN, United States, 4Department of Mechanical and Aerospace Engineering, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Multi-baseline proton resonant frequency (PRF) shift MR thermometry has been proposed to address the potential mismatch between baseline images and images during thermal therapy in moving organs. Previous methods had to compromise the spatial coverage to increase temporal resolution for resolving motion. This work developed a dynamic 3D stack-of-radial multi-baseline PRF MR thermometry method to monitor high-intensity focused ultrasound heating in moving tissue. The proposed method achieved stable and accurate thermometry with volumetric coverage and high spatiotemporal resolution, validated in a tissue motion phantom with temperature probe measurements.
Introduction
Proton resonance frequency (PRF) shift-based MR thermometry is widely used in MR-guided thermal therapies [1], such as high-intensity focused ultrasound (HIFU) [2], by calculating the phase differences between baseline images and images during heating. Multi-baseline approaches [3,4] have been proposed to address the mismatch between the baseline and images in moving organs. However, most of the studies only acquired a few 2D slices to maintain temporal resolution for resolving motion.Recently, dynamic 3D thermometry methods based on stack-of-radial MRI have achieved volumetric coverage with high spatiotemporal resolution [5-9]. These studies were performed in non-heating human subject scans and phantom heating experiments with limited or no motion. Therefore, 3D stack-of-radial thermometry needs to be further investigated during heating in tissue with relevant motion characteristics and reference temperature measurements.
In this study, we investigated a dynamic 3D stack-of-radial multi-baseline PRF MR thermometry method to monitor HIFU heating in moving tissue. We validated the motion tracking and temperature mapping performance in ex vivo tissue using a programmable MR-compatible motion stage and temperature probes.
Methods
Sequence and Reconstruction: Experiments were performed at 3T (Prisma, Siemens, Erlangen, Germany). An axial multi-echo golden-angle-ordered 3D stack-of-radial variable flip angle (FA) sequence [9] was used, with TR = 6.84ms, TE = [1.51, 2.78, 4.05, 5.32]ms, resolution = 1.3´1.3´5mm, FOV = 200mm, 18 slices, and FA =3° and 34°. During dynamic image reconstruction (Fig. 1A), a k-space weighted image contrast (KWIC) filter with 8/144 spokes in innermost/outermost regions was applied, followed by gradient calibration [10] and adaptive coil combination [11]. The magnitude images were echo-combined using sum-of-squares. The phase images were unwrapped along the echo direction and echo-combined [7] to an effective TE = 10ms.Motion Tracking and Multi-Baseline PRF Thermometry: 48 image frames (total duration of 26.6 seconds) before HIFU heating formed the multi-baseline library. Structural similarity index measure (SSIM) (Fig. 1B) was calculated between the magnitude image and the multi-baseline library. The 4 baseline images with the highest SSIM were combined using a weighted average to form a reference.
HIFU in a Tissue Motion Phantom: A research HIFU system (Image Guided Therapy, Bordeaux, France) with an 8-element annular array transducer at 2.5MHz operating frequency and 20W electrical power output was used. Baseline images were acquired for 60 seconds, followed by heating for 60 seconds and cool down for 7 min. We developed a programmable motion stage [12] (Fig. 2) consisting of a master actuator and an MRI-safe slave unit that drives the motion stage inside the scanner. The master/slave units were connected by fluid tubes, and the motion was transmitted to the slave unit via hydrostatic force. A pre-defined sinusoidal waveform with 20-second period and 18-mm range was used. An ex vivo pork tissue specimen was secured on the motion stage.
Analysis: The 3D PRF temperature change (ΔT) maps under static and pre-defined motion conditions were calculated on all slices at all time points, using single baseline and multi-baseline approaches. Mean absolute errors (MAE) were calculated between the PRF ΔT maps and the reference temperature.
Results
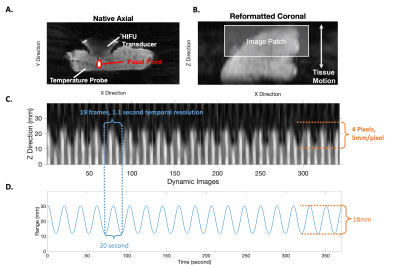
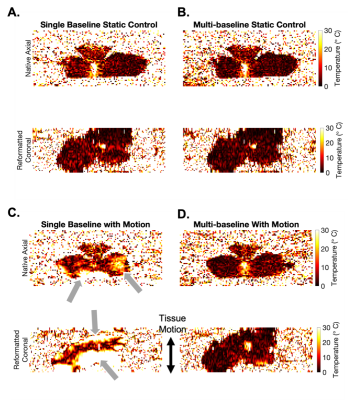
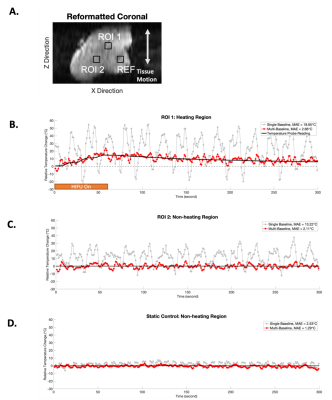
Figure 3A-B showed the image patch defined for motion tracking. Figure 3C shows the motion tracking plots. The periodicity and the range from image-based motion tracking matched the pre-defined sinusoidal waveform (Figure 3D). Figure 4 compares the PRF DT maps between the single baseline and the multi-baseline approaches during HIFU heating. Figures 4A-B show the results from static control conditions. Figures 4C-D show the results from heating during pre-defined motion. Figure 5 shows the ΔT measurement results. MAE from a heated region during motion was 18.65°C and 2.66°C for single baseline and multi-baseline approaches, respectively. MAE from a non-heated region was 13.22°C and 2.11°C for single-baseline and multi-baseline approaches, respectively.Discussion
This work is among the first to validate 3D dynamic MR thermometry in moving tissue during HIFU heating. Previous works [5-9] had to either compromise the spatial coverage to increase temporal resolution or lack motion tracking capability. Our proposed 3D stack-of-radial PRF thermometry achieved volumetric coverage (18 slices) with an in-plane resolution of 1.3mm and temporal resolution of 1.1 second/frame. While two FA were acquired to support T1-based thermometry, this work considered the data as a single stream for PRF thermometry.The performance of our proposed method was validated by both the motion tracking compared to pre-defined motion and the temperature probe measurements of HIFU heating. Our proposed method successfully captured both the motion frequency and range by utilizing image-based motion navigation. Our multi-baseline approach eliminated the phase errors induced by motion, and achieved MAE <3°C in both the heated and the non-heated regions, demonstrating accuracy and stability, respectively.
To better mimic the respiratory motion, actual breathing waveforms recorded from human subjects [12] can be programmed to our motion stage in future experiments, and a feature patch from additional orthogonal planes can be incorporated into our multi-baseline to achieve 3D motion tracking.
Conclusion
Our dynamic 3D stack-of-radial technique demonstrated stable and accurate multi-baseline PRF MR thermometry with volumetric coverage and high spatiotemporal resolution for monitoring HIFU heating in moving tissue. This work creates a basis for future in vivo studies of MR-guided thermal therapy in moving organs.Acknowledgements
This study was supported in part by Siemens Medical Solutions USA.References
[1 Rieke, Viola, and Kim Butts Pauly. “MR thermometry.” Journal of magnetic resonance imaging : JMRI vol. 27,2 (2008): 376-90. doi:10.1002/jmri.21265
[2] Holbrook, Andrew B., et al. "Real‐time MR thermometry for monitoring HIFU ablations of the liver." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 63.2 (2010): 365-373.
[3] Grissom, William A., et al. "Hybrid referenceless and multibaseline subtraction MR thermometry for monitoring thermal therapies in moving organs." Medical physics 37.9 (2010): 5014-5026.
[4] de Senneville, Baudouin Denis, et al. "Motion correction in MR thermometry of abdominal organs: a comparison of the referenceless vs. the multibaseline approach." Magnetic resonance in medicine 64.5 (2010): 1373-1381.
[5] Gaur, Pooja, and William A. Grissom. "Accelerated MRI thermometry by direct estimation of temperature from undersampled k‐space data." Magnetic resonance in medicine 73.5 (2015): 1914-1925.
[6] Zhang, Zhongshuai, Thomas Michaelis, and Jens Frahm. "Towards MRI temperature mapping in real time—the proton resonance frequency method with undersampled radial MRI and nonlinear inverse reconstruction." Quantitative imaging in medicine and surgery 7.2 (2017): 251.
[7] Svedin, Bryant T., et al. "Multiecho pseudo‐golden angle stack of stars thermometry with high spatial and temporal resolution using k‐space weighted image contrast." Magnetic resonance in medicine 79.3 (2018): 1407-1419.
[8] Svedin, Bryant T., Allison Payne, and Dennis L. Parker. "Simultaneous proton resonance frequency shift thermometry and T1 measurements using a single reference variable flip angle T1 method." Magnetic resonance in medicine 81.5 (2019): 3138-3152.
[9] Zhang, Le, et al. "A variable flip angle golden‐angle‐ordered 3D stack‐of‐radial MRI technique for simultaneous proton resonant frequency shift and T1‐based thermometry." Magnetic resonance in medicine 82.6 (2019): 2062-2076.
[10] Armstrong, Tess, et al. "Free‐breathing liver fat quantification using a multiecho 3 D stack‐of‐radial technique." Magnetic resonance in medicine 79.1 (2018): 370-382.
[11] Walsh, David O., Arthur F. Gmitro, and Michael W. Marcellin. "Adaptive reconstruction of phased array MR imagery." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 43.5 (2000): 682-690.
[12] Simonelli, James, et al. "Hydrostatic actuation for remote operations in MR environment." IEEE/ASME Transactions on Mechatronics 25.2 (2019): 894-905.
Figures
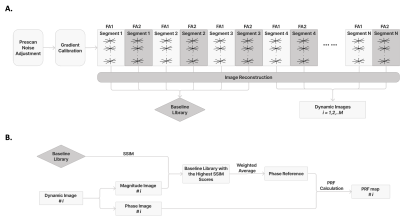
Figure 1: 3D Stack-of-Radial Sequence, Reconstruction and Multi-baseline PRF Method.
(A) Diagram of the multi-echo stack-of-radial variable flip angle (FA) sequence and image reconstruction. (B) Diagram of the multi-baseline PRF-based thermometry method.
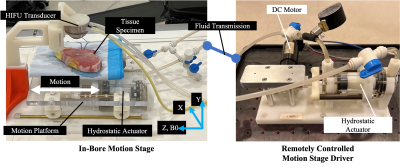
Figure 2: Programmable MRI-Compatible Motion Stage.
Setup of the motion stage with a master-slave actuator pair. The master actuator (right) in the MRI control room is powered by a DC motor, and the MRI-compatible in-bore slave actuator (left) drives a 1-degree of freedom (DOF) motion stage. The tissue specimen moves with the motion stage, while the HIFU transducer is fixed from above (does not move with the stage). Motion and the B0 magnetic field are both in the Z direction. A pre-defined motion waveform for the stage can be programmed and controlled remotely from the MRI control room.