4081
Addressing Acquisition Variability in DWI on Prostate Cancer with Unsupervised Methods
Batuhan Gundogdu1, Jay M Pittman2, Aritrick Chatterjee2, Milica Medved2, Roger Engelmann2, Aytekin Oto2, and Gregory S Karczmar2
1Radiology, University of Chicago, Chicago, IL, United States, 2University of Chicago, Chicago, IL, United States
1Radiology, University of Chicago, Chicago, IL, United States, 2University of Chicago, Chicago, IL, United States
Synopsis
Diffusion-weighted MR images are typically obtained as multiple acquisitions with multiple diffusion-sensitizing gradient directions. Due to molecular motion, some acquisitions suffer from signal loss at random locations. This affects cancer conspicuity and degrades the diagnostic efficacy of DWI. We propose an agglomerative clustering-based unsupervised method to address this. The model automatically rejects acquisitions of voxels that are likely to be corrupted by bulk motion and lack coherence with the rest of the acquisitions. We observed that this method both reduces the DWI signal variability and enhances the cancer detection accuracy.
Introduction
MRI is increasingly important for prostate cancer screening1. Diffusion-weighted images (DWI), and apparent diffusion coefficient (ADC) maps show restricted water diffusion, indicating a higher probability of cancer2,3. DWI and ADC maps have low signal-to-noise ratios (SNR), making it difficult to identify suspicious areas and to provide correct locations for targeted biopsy4. The typical approach to improve SNR is to collect multiple acquisitions during the scan and use their average as the MR image. However, this results in blurring due to motion between acquisitions. In addition, small bulk motions of the prostate occurring during application of diffusion sensitizing gradients can result in loss of signal and obscure regions with restricted diffusion. For high b values, this effect is more drastic since the DWI sequence becomes more sensitive to motion artifacts with increased b values, which also provide the greatest sensitivity to restricted diffusion associated with cancer. Thus, for each group of acquisitions, the signal for cancer voxels at high b values may include some relatively high signals due to restricted diffusion, and some lower signals that are likely to be artifacts due to local motion. In this case, conventional signal averaging is not appropriate since it combines acquisitions that show cancer signals with acquisitions that do not. The extent and adverse effects of this inter-acquisition variation have recently been quantitatively studied5. Although there are some studies aiming to address the signal variation via post-filtering and image enhancement techniques6, pre-selection of fruitful acquisitions on voxel-level remails an unsolved problem.Methods
In this study, we propose to address this issue via an unsupervised approach that rejects the potentially motion-corrupted acquisitions prior to image generation. Therefore, we assign more weight to the higher signals versus lower signals. The method we propose objectively filters the set of acquisitions to be used in image generation, as opposed to taking the mean of all acquisitions. Specifically, we apply hierarchical agglomerative clustering algorithm (HAC) over the set of acquisitions for each voxel and calculate the similarity ‘tree’ reflecting a measure of the coherence of acquisitions7. HAC pairs two most similar observations into a larger cluster at each step, potentially representing the true restricted diffusion in the voxel-of-interest. This pairing step continues until the whole set of acquisitions are segmented into two clusters. For merging clusters, we used the Ward’s minimum variance criterion8 so that minimum increase in the total within-cluster variance is obtained after merging. In the end, the cardinalities and the means of the resulting two clusters are compared to determine the acquisitions to be rejected. We hypothesize that higher signals among the set of acquisitions for a given voxel on DWI are likely to correctly represent restricted diffusion, while lower signals in the same voxel are more likely to be due to motion. Therefore, if the mean difference between the clusters is larger than the noise range, then this variation is more likely to be originating from a motion-induced signal loss, and accordingly, all acquisitions of the cluster with a smaller mean are rejected if it is not constituting at least 80% of the acquisitions. This method is based on the hypothesis that artifacts (predominantly local motion) in DWI are more likely to decrease signal than to increase signal. The resulting diffusion-weighted image is produced from the mean of the remaining acquisitions for each voxel. Figure 1 shows dendrogram trees from the HAC algorithm, calculated for a cancer voxel and the contralateral benign tissue.Results
The proposed algorithm was applied to DWI data from ten patients with biopsy verified prostate cancers. 12 or 24 acquisitions were obtained for each patient and the HAC algorithm was applied to discard corrupted acquisitions and reduce signal variability. HAC filtered out an average of 49% of the acquisitions. The ADC map with HAC-based filtering shows biopsy-verified cancer regions. Figure 2 compares the ADC maps from HAC-filtered images to the original mean image. HAC detects voxels with cancer that the mean image misses. Comparison of the standard deviation maps of the mean images with all acquisitions to the HAC-processed images shows that the undesired variation on the prostate region was mostly removed, and the signal standard deviation was reduced by an average of 42%. Standard deviation maps of two patients are given in Figure 3, with and without HAC-based filtering. The noise remaining in the image is close to the level expected from SENSE reconstruction of electronic white noise.Discussion
Inter-acquisition variability in DWI is large relative to differences between prostate cancer and normal tissue5. Taking the conventional mean of multiple acquisitions to improve SNR disregards the effects of this variation and can obscure cancers. Here, we propose a new approach to filtering diffusion-weighted images to reduce the effects of motion-induced signal loss via unsupervised clustering. This decreases signal variability and increases cancer conspicuity.Conclusion
We propose an unsupervised method of addressing the motion-induced inter-acquisition variability in DWI. We show that hierarchical clustering of acquisitions increases cancer conspicuity. In the future, this method will be extended to include information from neighboring voxels in the clustering algorithm and used as input to multi-image super-resolution methods.Acknowledgements
This work was supported by the Sanford J. Grossman Charitable Trust (Sanford J. Grossman Center of Excellence in Prostate Imaging and Image-Guided Therapy)References
- Steiger, P. & Thoeny, H. C. Prostate MRI based on PI-RADS version 2: How we review and report. Cancer Imaging 16, 1–9 (2016).
- Haffner, J. et al. Peripheral zone prostate cancers: Location and intraprostatic patterns of spread at histopathology. Prostate 69, 276–282 (2009).
- Reissigl, A. et al. Frequency and clinical significance of transition zone cancer in prostate cancer screening. Prostate 30, 130–135 (1997).
- Saritas EU, Lee JH, Nishimura DG. SNR dependence of optimal parameters for apparent diffusion coefficient measurements. IEEE Trans Med Imaging. Feb;30(2):424-37 (2011)
- Jay M. Pittman et al. Directional and Inter-acquisition Variability in DWI (DAVID), ISMRM (2021)
- Descoteaux M. et al. Impact of Rician adapted Non-Local Means filtering on HARDI. Med Image Comput Comput Assist Interv. 2008;11(Pt 2):122-30. doi: 10.1007/978-3-540-85990-1_15. PMID: 18982597.
- Müllner, Daniel. Modern hierarchical, agglomerative clustering algorithms. CoRR abs/1109.2378 (2011)
- Murtagh, F., Legendre, P. Ward’s Hierarchical
Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J
Classif 31, 274–295 (2014)
Figures
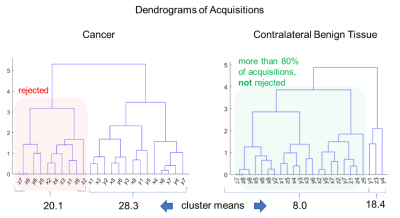
Figure 1: Dendrogram trees of HAC calculated on cancer
and benign tissue. Note that the HAC algorithm detects all acquisitions on
gradient direction ‘Z’ and some acquisitions of ‘X’ and ‘Y’ to be discarded.
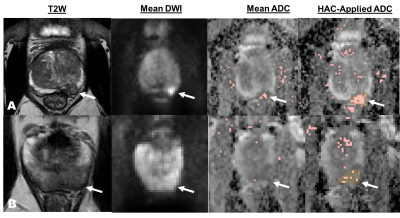
Figure 2: T2W MRI, DWI, and ADC
maps of two patients. The HAC algorithm increases cancer conspicuity in ADC
maps. The marked regions denote voxels with ADC values less than 1450 um2/sec.
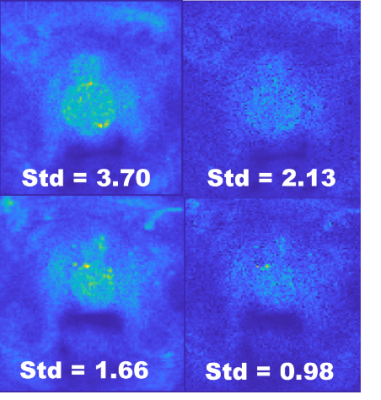
Figure 3: Standard deviation maps
of two patients, without (left) and with (right) the proposed
filtering. Note that the HAC-based
filtering reduces the standard deviation on the prostate region, making it
close to the electronic white noise in the image.
DOI: https://doi.org/10.58530/2022/4081