4069
Subject-specific Analysis for Diffusion and Kurtosis Changes in mild Traumatic Brain Injury (mTBI) Patients1GE Research, Niskayuna, NY, United States, 2GE Healthcare, Niskayuna, NY, United States, 3Walter Reed National Military Medical Center, Bethesda, MD, United States, 4Uniformed Services University of the Health Sciences, Bethesda, MD, United States
Synopsis
Advanced neuroimaging capabilities enabled by the MAGNUS (ultra-high performance head gradient) system was leveraged in this study to assess subject-specific microstructural changes with acute and chronic mild traumatic brain injury. MAGNUS allows for a four-fold increase in maximum gradient-amplitude compared to current clinical whole-body scanners and enables identification of new imaging biomarkers for improved detection of microstructural changes in the brain. We present an outlier analysis approach that allows study of microstructure differences in individual subjects rather than solely on a group basis. Preliminary results demonstrate identification of several white-matter regions in patient groups that differ from healthy controls.
Introduction
Mild traumatic brain injury (mTBI) is a heterogenous disease presenting significant challenge to the discovery of disease-sensitive imaging biomarkers1. Impact acceleration forces are the leading cause for TBI and have deleterious effects on the brain manifesting as a spectrum of anatomical damage such as axonal swelling, myelin disruption, contusion with torsion, compression and tension in white-matter tracks2,3. These microstructural changes are not typically evident via conventional MR/CT anatomical imaging and are interrogated via diffusivity metrics2,4–7 to assess microstructural integrity, which is dependent on system gradient performance.The ability to generate sufficiently high b-value diffusion encoding with sufficient signal-to-noise ratio (SNR), and thereby the sensitivity of measurement to diffusion processes, is dependent on the performance of the gradient sub-system. Critically high gradient amplitudes are highly desirable for diffusion-MRI, given the quadratic scaling of b-values with Gmax while allowing for shorter diffusion encoding pulse-widths thus moving closer towards the narrow pulse approximation. On the other hand, higher slew rates maximize waveform efficiency and lead to shorter EPI readout echo spacing, minimizing image distortion and maximizing SNR efficiency. The MAGNUS8 (Microstructure Anatomy Gradient for Neuroimaging with Ultrafast Scanning) asymmetric head-only gradient system delivers simultaneously Gmax = 200 mT/m and SRmax = 500 T/m/s, with a 1 MVA gradient driver, with the design allowing for higher PNS thresholds than with whole-body gradient systems9.
In this study, we leverage MAGNUS gradients in an ongoing clinical study of acute and chronic mTBI for a multi-shell acquisition protocol with bmax=4000 s/mm2 for greater sensitivity to kurtosis10. Further, we present an outlier analysis approach that leverages data from normal subject to highlight subject-specific differences, which enables its use in clinical practice.
Methods
Acquisition: A total of 11 subjects (1 acute-mTBI, 3 chronic-mTBI, 7 healthy) were scanned twice under an IRB-approved protocol on a 3T MRI MAGNUS system (Figure 1). A 32-channel phased array head coil (NOVA Medical, Wilmington, MA) was used for all acquisitions. A multi-shell acquisition was utilized with q-vectors distributed such that the sampling pattern minimized the energy of electrostatic repulsion for points on a sphere11, for b-values of 500 (npoints=30), 2000 (npoints=40), and 4000 (npoints=50) s/mm2. Five T2-weighted (b=0) images were also included in the multi-shell acquisition. For TE/TR = 44.2/7000ms, 160x160x86 matrix, diffusion data were acquired with 1.5-mm isotropic resolution for an overall acquisition time of 15 mins. Additionally, a T1-weighted MPRAGE image was also acquired for co-registration.Reconstruction: Diffusion weighted images were corrected for gradient non-linearity, eddy current distortion, bulk motion and susceptibility in our custom reconstruction pipeline12,13. Data was denoised using Generalized Spherical Deconvolutions (GenSD)14,15. Diffusion and kurtosis tensors were fitted using a non-negativity constrained least-squares approach to compute associated metrics.
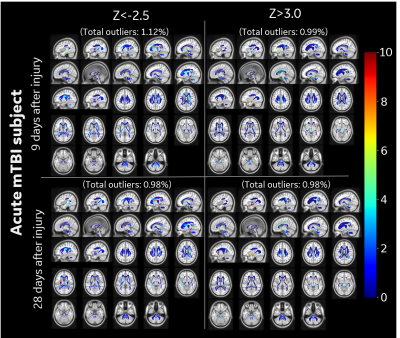
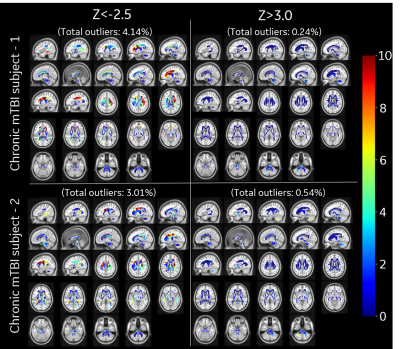
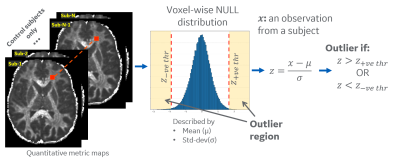
Outlier Analysis: All diffusion metrics were mapped to MNI atlas space using our joint T1-FA co-registration for accurate mapping16. White matter region was parcellated into a custom 76 anatomical ROIs derived from ICBM-DTI-81 atlas17. For outlier analysis, a voxel-wise null-distribution was computed in a common MNI space for diffusion metrics from healthy controls (Figure 2), which included diffusion and kurtosis tensor metrics such as, orthogonal kurtosis (K-Orth), fractional anisotropy (FA), etc. The null-distribution was summarized by mean (μ) and standard deviation (σ) values at each voxel, for each metric. Subject-specific distance from this null-distribution is measured by computing voxel-wise z-scores (z=(x-μ)/σ) for an observation x. Z-score thresholds of +3.0 (increased value) and -2.5 (reduced values) were selected to define voxels as outliers. The percentage of outlier voxels in each anatomical ROI was calculated to analyze differences for each subject.
Results and Discussion
In this study outlier analysis for subject specific metrics was demonstrated over three representative populations (healthy, acute mTBI and chronic mTBI) with K-Orth and FA metrics. Distribution of K-Orth outlier percentages for control subjects show very few outliers (<0.3%) throughout the white matter region for both upper and lower thresholds (Figure 3). The acute subject showed larger outlier percentage in several regions including external capsule and cingulum regions on first visit (9-days post injury) as well as second visit (28 days post injury), albeit with lower outlier count (Figure 4), potentially indicating downstream diffuse injury impacts, biological response (reaction) to insult and/or biophysical remodeling. Chronic subjects showed largest percentage of outliers across several white matter regions (Figure 5), which is potentially indicative of their symptomatic presentation even after >90-days post-injury. Interestingly, in contrast to the acute patient, chronic mTBI patients showed 6-20 times more outliers in lower-side than upper-side of K-Orth, which might indicate differences in disease progression differences. Outlier analysis with FA metric also demonstrated similar trend in all subjects (not shown in figures). Future study will include larger sample size for more accurate modeling control distribution.Conclusion
Microstructure sensitive diffusion MRI is a promising approach to study mTBI. Even with limited sample-size, especially control subjects, this initial study highlights identification of sub-regions differing from the healthy control group and therefore likely to be affected by mTBI. Further, our outlier analysis approach allows observation of changes in individual subjects unlike commonly used group analysis. This presents the possibility of identification of diagnostic and prognostic markers of mTBI that use both subject specific and group wise information with high clinical value.Acknowledgements
Grant funding from NIH U01EB028976, CDMRP W81XWH-16-2-0054.
The opinions or assertions contained herein are the views of the authors and are not to be construed as the views of the U.S. Department of Defense, Walter Reed National Military Medical Center, or the Uniformed Services University.
References
1. Incidence, risk factors and prevention of mild traumatic brain injury: results of the who collaborating centre task force on mild traumatic brain injury. http://www.medicaljournals.se/jrm/content/abstract/10.1080/16501960410023732 doi:10.1080/16501960410023732.
2. Palacios, E. M. et al. White Matter/Gray Matter Contrast Changes in Chronic and Diffuse Traumatic Brain Injury. Journal of Neurotrauma 30, 1991–1994 (2013).
3. Palacios, E. M. et al. The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Science Advances 6, eaaz6892.
4. Shenton, M. et al. A Review of Magnetic Resonance Imaging and Diffusion Tensor Imaging Findings in Mild Traumatic Brain Injury. Brain Imaging Behav 6, 137–192 (2012).
5. Niogi, S. N. & Mukherjee, P. Diffusion Tensor Imaging of Mild Traumatic Brain Injury. The Journal of Head Trauma Rehabilitation 25, 241–255 (2010).
6. Arfanakis, K. et al. Diffusion Tensor MR Imaging in Diffuse Axonal Injury. AJNR: American Journal of Neuroradiology 23, 794 (2002).
7. Zhuo, J. et al. Diffusion Kurtosis as an In Vivo Imaging Marker for Reactive Astrogliosis in Traumatic Brain Injury. Neuroimage 59, 467–477 (2012).
8. Foo, T. K. F. et al. Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magnetic Resonance in Medicine 83, 2356–2369 (2020).
9. Tan, E. T. et al. Peripheral nerve stimulation limits of a high amplitude and slew rate magnetic field gradient coil for neuroimaging. Magnetic Resonance in Medicine 83, 352–366 (2020).
10. Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H. & Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine 53, 1432–1440 (2005).
11. Dk, J., Ma, H. & A, S. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic resonance in medicine vol. 42 https://pubmed.ncbi.nlm.nih.gov/10467296/ (1999).
12. Tan, E. T., Marinelli, L., Slavens, Z. W., King, K. F. & Hardy, C. J. Improved correction for gradient nonlinearity effects in diffusion-weighted imaging. Journal of Magnetic Resonance Imaging 38, 448–453 (2013).
13. Bhushan, C. et al. Co-registration and distortion correction of diffusion and anatomical images based on inverse contrast normalization. Neuroimage 115, 269–280 (2015).
14. Sperl, J. I. et al. Model-based denoising in diffusion-weighted imaging using generalized spherical deconvolution. Magn Reson Med 78, 2428–2438 (2017).
15. Abad, Nastaren, Marinelli, Luca, Madhavan, Radhika & Foo, TKF. Model Based Denoising of Diffusion MRI Reduces Bias in Tensor Derived Parameters and Connectivity Measures. in Proceedings for the International Society for Magetic Resonance in Medicine (2021).
16. Quachtran, B., Joshi, A. A., Bhushan, C., Leahy, R. M. & Shattuck, D. W. Combined T1-Diffusion MRI Registration. OHBM 2018
17. Li, X. et al. Single-subject Voxel-based Analysis for mTBI using Multi-shell Diffusion MRI. OHBM
Figures

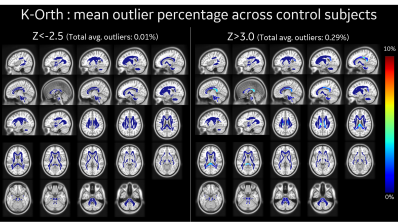
ROI-wise mean outlier percentage across all control subjects (N=7), as identified by (left) z-ve thr=-2.5, and (right) z+ve thr=3.0 in our outlier analysis approach with orthogonal kurtosis measure. Almost all ROIs show very few outliers (<3%) in control subjects, with total average outlier of <0.29% across white-matter region.