4067
Development and robust analysis of 3D fat-suppressed T2 MRI for head and neck radiotherapy treatment planning1Radiation Oncology, MD Anderson Cancer Center, Houston, TX, United States, 2Philips Healthcare, Best, Netherlands, 3Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States, 4Radiation Physics, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
In order to improve head and neck radiotherapy treatment planning, a series of 3D fat-suppressed T2-weighted sequences were developed with varying pulse sequence parameters. One non-fat-suppressed and five fat-suppressed sequences were acquired on five patients, and on each image, four structures were segmented by six radiation oncology physicians (observers). Robust and comprehensive analysis was performed to assess qualitative and quantitative image quality metrics. This included geometric distortion, SNR and CNR measurements, structure conspicuity, interobserver segmentation variability, and qualitative image quality rankings. The results of these were used to determine the optimal fat-suppressed sequence for head and neck radiation treatment planning.
Introduction
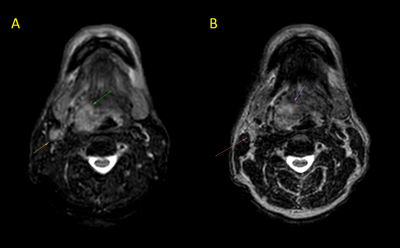
The use of MRI in radiation treatment planning has drastically increased over the last several years1. This is due to the superior soft tissue contrast produced by MRI compared to CT, which has conventionally been used in treatment planning. For MR images to be clinically useful for treatment planning, they must possess minimal distortion, high resolution in three dimensions, and high contrast-to-noise (CNR) in tumors and organs-at-risk. While conventional 3D T2-weighted (T2w) MRI sequences have been successfully implemented for treatment planning, head and neck tumors and surrounding structures are often obscured by nearby hyperintense fat signal, which can lead to inaccurate segmentation and suboptimal dose delivery (Fig. 1). Therefore, we sought to develop a clinically feasible fat-suppressed T2 sequence acquired on MR-Simulation and MR-Linac devices. Furthermore, iterations of this sequence were comprehensively analyzed to identify the optimal pulse sequence parameters for final use in head and neck radiotherapy treatment planning.Methods
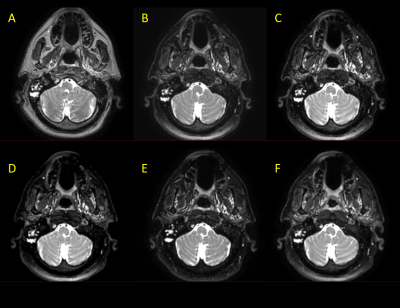
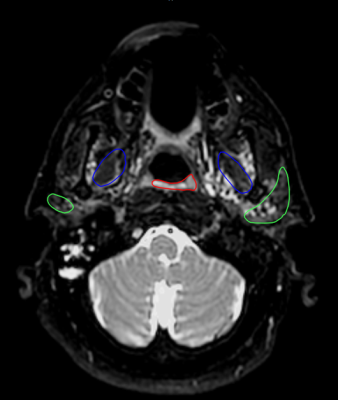
From preliminary work that investigated basic Short-TI Inversion Recovery (STIR), Spectral Attenuated Inversion Recovery (SPAIR), and Dixon acquisitions on an MR-Linac device2, the SPAIR technique was chosen for further development due to its compromise of scan time and signal-to-noise (SNR). Several iterations of a 3D SPAIR T2w sequence were developed by adjusting relevant pulse sequence parameters such as TR, TE, refocusing angle, bandwidth, FID reduction, echo train length, and oversample factor. After eliminating iterations which produced severe artifacts, poor fat suppression, or poor SNR, five final 3D T2w SPAIR sequence iterations were chosen for further analysis (Fig. 2). With appropriate informed consent and IRB approval, images were acquired with these sequence iterations on five head and neck cancer patients on an MR-Linac device. The images acquired from these sequence iterations were subjected to comprehensive and robust analysis. Overall geometric distortion was assessed by registering the images to their corresponding T2 images, autosegmenting the outline of the head and neck regions, and computing the Dice similarity coefficient (DSC) and Hausdorff distance (HD). Each of the images were contoured by six independent radiation oncology physicians (observers). Four structures were segmented: tumor GTV, suspicious lymph nodes, left/right parotid glands, and left/right pterygoid muscles (Fig. 3). SNR and CNR measurements (relative to muscle and fat) were performed on these segmentations. Furthermore, the visibility of these structures was quantified through conspicuity measurements (a metric combining contrast and surrounding signal complexity3), which was automated through software developed through this project. The ability of these sequences to allow for accurate and precise contouring was assessed by performing interobserver variability analysis on the segmentations. For each structure, DSC and HD were calculated pairwise between each observer for a given image and averaged. Follow-up simultaneous truth and performance level estimation (STAPLE) analysis was also performed to further assess interobserver variability4. Lastly, qualitative rankings for each image were provided by each of the observers and two separate imaging physicists which encompassed presence of artifacts, level of fat suppression, and clarity of structures.Results
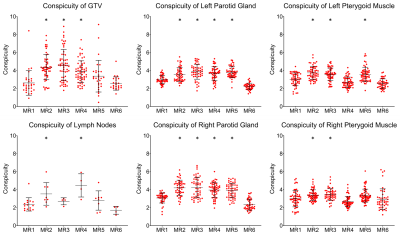
The non-fat-suppressed 3D T2w image and five 3D T2w SPAIR images were successfully acquired on each of the five patients. Segmentations of the four structures (tumor GTV, suspicious lymph nodes, left/right parotid glands, and left/right pterygoid muscles) were segmented by the observers. These observers, along with two imaging physicists have provided qualitative image quality rankings. In-house software was developed to automatically extract signal values 2 pixels inside and outside each point of the segmentation boundaries. The resultant conspicuity values per slice occupied by the structure was calculated, and these values from one observer’s segmentations on each image from one patient are plotted in Fig. 4. Of note, conspicuity of MR2 is significantly high across all structures, MR3 and MR5 in normal structures, and MR4 in malignant structures. In contrast, conspicuity of MR1 (non-fat-suppressed 3D T2w) and MR6 are consistently low in all structures. Conspicuity measurements from the remaining observers and remaining patients will be presented, along with the results of geometric distortion, SNR and CNR measurements, qualitative image quality rankings, and interobserver variability analysis.Discussion and Conclusion
Deliverables from this project are two-fold. First, an optimized 3D T2w SPAIR sequence has been developed and validated for acquisition on MR-Linac devices for head and neck radiotherapy treatment planning purposes. Conspicuity measurements demonstrate that all measured structures are less visible in the non-fat-suppressed 3D T2w compared to its SPAIR counterparts. The sequence will be shared amongst various other institutions with MR Simulators and MR-Linacs as part of multi-institutional validation and standardization. Second, a robust and comprehensive analysis platform for image acquisition optimization has been developed. The combination of qualitative and quantitative metrics of image quality and segmentation precision have been incorporated into an image grade and can be applied to any type of imaging technique for optimization. The culmination of these deliverables has led to improved radiation treatment planning in head and neck cancer cases at our institution.Acknowledgements
Supported by The University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences TL1 Program (TL1 TR003169)References
1. K. M. Jones, K. A. Michel, J. A. Bankson, C. D. Fuller, A. H. Klopp, and A. M. Venkatesan, “Emerging Magnetic Resonance Imaging Technologies for Radiation Therapy Planning and Response Assessment,” Int. J. Radiat. Oncol., vol. 101, no. 5, pp. 1046–1056, Aug. 2018, doi: 10.1016/j.ijrobp.2018.03.028.
2. F. Del Grande et al., “Fat-Suppression Techniques for 3-T MR Imaging of the Musculoskeletal System,” RadioGraphics, vol. 34, no. 1, pp. 217–233, Jan. 2014, doi: 10.1148/rg.341135130.
3. G. Revesz, H. L. Kundel, and M. A. Graber, “The Influence of Structured Noise on the Detection of Radiologic Abnormalities,” Invest. Radiol., vol. 9, no. 6, pp. 479–486, Nov. 1974, doi: 10.1097/00004424-197411000-00009.
4. S. K. Warfield, K. H. Zou, and W. M. Wells, “Simultaneous Truth and Performance Level Estimation (STAPLE): An Algorithm for the Validation of Image Segmentation,” IEEE Trans. Med. Imaging, vol. 23, no. 7, pp. 903–921, Jul. 2004, doi: 10.1109/TMI.2004.828354.
Figures