4060
In vivo phase-incrementing SEE-HSelMQC method to resolve tumor biomarker images by synchronizing RF phase increments and phase-encoding steps1Center for Animal MRI, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Biomedical Research Imaging Center (BRIC), University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Department of Radiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4Department of Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
We have developed the phase-incrementing MRSI (pi-MRSI) method to resolve overlapping biomarker images in the presence of a frequency encoding gradient during acquisition time. We report here the pi-SEE-HSelMQC experiments on a pre-clinical Bruker 9.4T spectrometer. The choline-selective and lactate CH-selective RF pulses in the pulse sequence were phase incremented by 10° in opposite signs in synchronization with the phase-encoding steps. In vivo two-dimensional pi-SEE-HSelMQC imaging of lactate and choline acquired from the PC3 human prostate cancer xenograft in a nude mouse showed opposite image offsets, shifted away from spurious signals. The pi-SEE-HSelMQC method completely suppresses lipid and water.
Introduction
In MR spectroscopic imaging (MRSI), magnetic field gradients are not often applied during data acquisition time to avoid image overlapping of different biomarkers and spurious signals including unsuppressed residual water and lipids. Chemical Shift Imaging (CSI), the monumental MRSI development, replaces the frequency encoding gradient with an additional dimension of phase encoding.1 Therefore, the CSI experiments require substantially longer scan time than MRI. Previously, we have developed the selective multiple-quantum coherence transfer (Sel-MQC),2 the spin-echo enhanced Sel-MQC (SEE-SelMQC),3 and the Molecular-Specific Coherence Sel-MQC (MSC-SelMQC)4 methods for spectroscopic imaging in tissues containing high level of mobile lipid. These methods have employed multidimensional CSI phase-encoding schemes for multi-voxel mapping of biomarker spatial distributions with excellent water and lipid suppression. Metabolites,2,3 anti-neoplastic agents,5 and polyunsaturated fatty acids (PUFA)6 were successfully detected in mouse tumor models, healthy human breast tissues and human breast cancer. To speed up the Sel-MQC spectroscopic imaging,7 we have replaced the CSI phase-encoding gradients with a spiral k-space mapping scheme, achieving about 150 fold increase of PUFA imaging speed. Nevertheless, the spiral gradients were applied during data acquisition time in the Spiral-SelMQC method, in which the biomarkers can only be imaged one at a time to avoid biomarker signal overlapping. To augment efficiency, we have recently developed the novel phase-incrementing MRSI (pi-MRSI) approach for fast imaging of multiple metabolites. Here we report results from the pi-SEE-HSelMQC method, modified from the SEE-SelMQC CSI sequence3 by replacing a phase encoding dimension with the frequency-encoding gradient. The overlapping biomarker images were resolved by synchronized RF phase incrementing and phase encoding steps.Methods
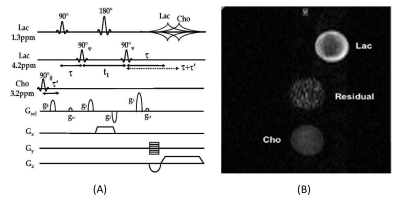
The pi-MRSI principle was demonstrated in phantoms and in vivo using mouse tumor models on a Bruker 9.4T BioSpec 94/30USR MRI spectrometer (AVII PV 6.0.1) for multi-biomarker imaging. The system has a BFG-240/120-S13B shielded gradient (12cm bore size) with the maximum gradient of 999.63, 1,001.9, and 1,001.6 mT/m in x-, y-, and z-directions, respectively. In the pi-SEE-HSelMQC method (Fig. 1A), a read-gradient was applied for lactate (or PUFA) and choline imaging, similar to that in a classical MRI experiment. The slice selection was achieved using a slice-selective 180° pulse, similar to the original SEE-SelMQC method.3 To synchronize with the phase encoding steps, the RF phases of the choline-selective 90° pulse (θ) at 3.2ppm and the lactate-CH selective 90° pulse (ψ) at 4.2ppm for MQ transfer to SQ modes [or alternatively the lactate CH-selective 90° pulse (φ) for MQ-preparation] were incremented by -10° and 10°, respectively. This introduced opposite image offsets to lactate and choline signals and resolved image overlapping of the two biomarkers, away from the residual water and lipid signals.Results
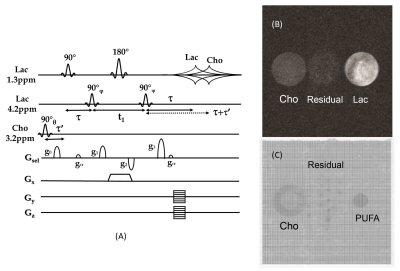
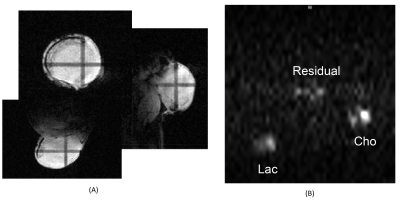
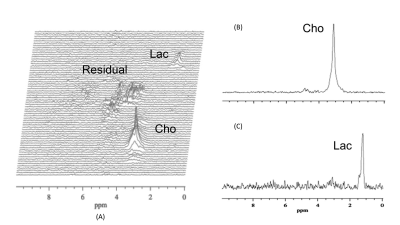
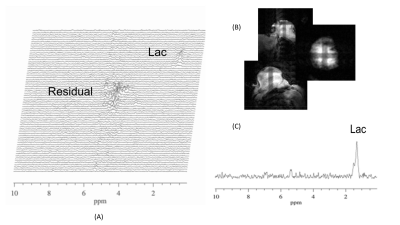
The 2D pi-SEE-HSelMQC images of choline and lactate were obtained from fresh Dannon whole milk yogurt in a 50 mL conical tube using the Bruker 35 mm quadrature volume coil. Opposite choline and lactate imaging offsets were introduced in the phase-encoding direction, shifting choline and lactate images away from the residual water in the image center (Fig. 1B). Bandwidth = 10 kHz; FOV = 128 x 144 mm2; image matrix size = 128 x 72. T1 relaxation delay = 1.5s. The pi-MRSI scan took 1.48 min. We have also carried out the corresponding pi-SEE-SelMQC CSI experiments (Fig. 2A) to observe the imaging offsets introduced to lactate (or PUFA) and choline signals (Fig. 2B-C). Next, we performed in vivo 2D pi-SEE-HSelMQC imaging with the frequency-encoding read-gradient (Fig. 1A) on a PC3 prostate tumor grown in the right flank of nu/nu mouse (Fig. 3A). The tumor lactate and choline images were detected with opposite image offsets away from the image center (Fig. 3B), due to -10° and 10° phase increments of the choline selective RF pulse and the last lactate CH selective RF pulse, respectively, in synchronization with the phase encoding steps. The residual water and lipid signals stay at the image center, not overlapping with the resolved lactate and choline images. Tumor size: 16.05 mm x 12.50 mm x 8.31mm. Bandwidth = 5000Hz. Image matrix size = 32 x 54, FOV = 90 x 90mm2. The gradient ratio g0: g1: g2: g3 = 12%: 0: -12%: 24% and gcr = 5%, with an equal gradient duration of 1 ms. In an in vivo 1D pi-SEE-HSelMQC CSI experiment, an orthotopic MDA-MB-231 human mammary adenocarcinoma xenograft tumor was studied with a home-made RF gap resonator fitting the tumor size of 13.15mm x 10.61mm x 9.19mm. The tumor lactate and choline spectra presented opposite image offsets in the phase encoding direction, away from the residual spurious water signal at the image center (Fig. 4). The choline-selective and MQ-coherence gradient ratio was g0: g1: g2: g3 = 15%: 0: -15%: 30%. In a control experiment, we set g0: g1: g2: g3 = 0: 0: -15%: 30% without refocusing choline signal. Only lactate was detected in the control experiment (Fig. 5).Conclusion
By synchronizing the biomarker-selective RF phase increments and the spatial phase encoding steps, the pi-MRSI method has resolved overlapping biomarker MR images in the presence of read gradient. The novel fast pi-MRSI imaging of multiple biomarkers with 3D Cartesian and non-Cartesian k-space mapping have many potential clinical applications in human disease diagnosis and monitoring therapeutic interventions.Acknowledgements
We thank the funding support from Linebarger Comprehensive Center (P30 CA016086), Bowles Center for Alcohol Studies (P60 AA011605), and Carolina Institute for Developmental Disabilities (U54 HD079124) for Center for Animal MRI facilities at UNC-CH. We are grateful to the Xsos imaging analysis program provided by Dr. Diokma C. Shungu from the Weill Cornell Medical College, New York City, NY.References
1. Brown TR, et al., “NMR chemical shift imaging in three dimensions,” Proc Natl Acad Sci U S A. 1982 Jun; 79 (11):3523-26.
2. Qiuhong He, et al, “Single Scan In Vivo Lactate Editing with Complete Lipid and Water Suppression by Selective Multiple Quantum Coherence Transfer with Application in Tumors,” J. Magn. Reson., Series B 106, 203-11 (1995).
3. Qiuhong He et al., “Proton Detection of Choline and Lactate in EMT6 Tumors by Spin-Echo-Enhanced Selective Multiple-Quantum-Coherence Transfer,” J. Magn. Reson., Series B 111, 18-25 (1996).
4. Qiuhong He, “Simultaneous mapping of multiple chemicals with suppression of unwanted signals via molecular specific coherence (MSC)-SelMQC (selective multiple quantum coherence).” U.S. Patent No. 9,285,443.
5. Qiuhong He, et al., “Proton NMR Observation of the Antineoplastic Agent Iproplatin In Vivo by Selective Multiple Quantum Coherence Transfer (Sel‐MQC),” Magn. Reson. Med. 33, 414-16 (1995).
6. Qiuhong He, et al., “In vivo MR spectroscopic imaging of polyunsaturated fatty acids (PUFA) in healthy and cancerous breast tissues by selective multiple‐quantum coherence transfer (Sel‐MQC): A preliminary study,” Magn. Reson. Med. 58, 1079-1085 (2007).
7. Zhu, et al., “The fast spiral‐SelMQC technique for in vivo MR spectroscopic imaging of polyunsaturated fatty acids in human breast tissue,” Magn. Reson. Med. 67, 8-19 (2012).
Figures