4039
Is the tremor relief in Parkinson’s disease ancillary benefits during the ablation of thalamic Vim using MR guided focused ultrasound (MRgFUS)?1Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 2Center for Neurological Restoration, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Essential tremor (ET) and tremor dominant Parkinson’s disease (PD) treatment using MRgFUS share the same therapeutic target and the thermal dose threshold to create a thalamic lesion at ViM. However, PD patients respond to treatment typically only during the last few sonications, whereas ET patients respond earlier, typically within the first few sonications. Furthermore, we confirmed that zona incerta (ZI) is commonly affected due to proximity to ViM, by using postoperative T2-weighted images fused with auto-segmented ViM and ZI, demonstrating a possible causal relationship between the lesion overlap with ZI that primarily reduces tremor reduction in PD patients.
Purpose
MRgFUS is a non-invasive surgical option FDA-approved for medication refractory essential tremor (ET) and tremor dominant Parkinson’s disease (PD) patients, using thalamic ViM as the currently approved target for both disorders. The MR thermometry is essential in non-invasive MRgFUS ablation to verify the accurate treatment location and measure the temperature at the focus. Therefore, the purpose of the first few sonications with low temperature is to confirm alignment to the target Vim. Then, the treatment effect is verified using a reversible higher temperature to monitor the tremor relief with an accelerometer. After verification, a permanent lesion is created using temperatures above the ablative level (<55ºC). During the course of multiple sonications, from low to high power, the pattern of tremor reduction (as measured with an intraoperative accelerometer) was different between ET and PD patients. A possible reason is that while ViM is naturally heated first, adjacent tissue is heated secondarily, and other nuclei within this region may confer more benefit to PD tremor than ViM. The thermal dose is currently used to estimate tissue damage, and the effective dose area is based on the time the tissue is exposed to more than 43ºC, known as cumulative equivalent minutes at 43ºC (CEM43). The device software provides the dose region segmentation at two different thresholds of thermal dose of 17 CEM431 and 240 CEM432 equals tissue exposure at 43 ºC for cumulative 17 minutes and 240 minutes, respectively. We hypothesize to correlate a thermal dose measure at each sonication to tremor reduction as measured by intraoperative accelerometry. We further hypothesize that this correlation will be different for ET vs PD patients, and that causative explanations are due to alternative nuclei that affect PD tremor more than ET tremor.Methods
MRgFUS at the Cleveland Clinic uses ExAblate® 2000 (InSightec Inc., Tirat Carmel, Israel) integrated with Siemens Skyra 3T MRI. 5 ET and 7 PD patients were subject to intraoperative accelerometer recording from their finger, arm, and forearm after prior informed consent. The postural tremor from patients was recorded after each sonication while performing finger-to-chin positioning. The tremor amplitude calculated by root mean square (RMS) was normalized by pre-HIFU baseline recorded when patients were in a supine position inside the MRI. Thalamic ViM and zona incerta (ZI) was auto-segmented using Brainlab Elements software (Brainlab, Munich, Germany) on the preoperative MR imaging and then fused with postoperative day 1 T2 lesion volume (Fig 1A,B) to measure final lesion overlap with adjacent ZI, a well-established target for PD in DBS3. The accumulated dose area was acquired from MR thermometry, and the MRgFUS system provides colored overlays at two thresholds: 17 CEM43 (light blue) and 240 CEM43 (dark blue) (Fig 2C,D,E). We further confirmed/quantified the partial overlap between the accumulated dose areas with ZI along each sonication by measuring the distance in the inferior direction from the target focus on the coronal plane (Fig 1B).Results
There was no statistical difference in tremor reduction in ET patients between when the 17 CEM43 dose area was contained within the ViM and when it started to partially overlap with ZI (p=0.26), whereas in PD patients, statistically reduced tremor amplitude began when 17 CEM43 dose area started to create overlap with ZI (p=0.01, Fig 3A). When Comparing ET and PD patients, we observed a significant difference in their tremor reduction (%) when the 17 CEM43 dose coverage was limited to ViM (p=0.005, Fig 3A). In PD patients, a strong negative correlation was found between thermal dose threshold and the tremor reduction (%): r = -0.80 for 17 CEM43 and r = -0.72 for 240 CEM43. In addition, the follow-up tremor evaluation also showed a significant difference in CRST functional disability scores between ET and PD patients (ET: 4.33 ± 0.85, PD: 9.666 ± 2.31, p=0.01) although, the number of adverse events was not statistically meaningful (ET: 3.14 ± 0.35, PD: 4.44 ± 1.36, p=0.20).Conclusion
Tremor reduction in PD correlates better with the 17 CEM43 than conventional 240 CEM43, which is the thermal dose threshold for creating a permanent lesion. The timing for significant tremor reduction in PD patients coincides with when the dose area covered by 17 CEM43 starts to overlap with ZI (ViM lesion extend to ZI 100%) and then reaches a plateau (Fig 3C). PD patients have shown less efficient intraoperative treatment response and less durable tremor control (indexed by their follow-up CRST scores). Our findings suggest that when using MRgFUS, ZI has therapeutic potential for alleviating Parkinsonian tremor in PD patients. An improvement of the treatment efficacy can be made in PD by expanding options for thermal dose threshold and the therapeutic targets beyond the parameters for ViM ablation because PD is more than a tremor and patients can be benefited from rigidity, bradykinesia, and other PD-related pathological symptoms.Acknowledgements
No acknowledgement found.References
1. McDannold NJ, Vykhodtseva NI, Hynynen K: Microbubble Contrast Agent with Focused Ultrasound to Create Brain Lesions at Low Power Levels: MR Imaging and Histologic Study in Rabbits. Radiology 241:95–106, 2006 Available: https://doi.org/10.1148/radiol.2411051170.
2. Meshorer A, Prionas SD, Fajardo LF, Meyer JL, Hahn GM, Martinez AA: The effects of hyperthermia on normal mesenchymal tissues. Application of a histologic grading system. Arch Pathol Lab Med 107:328–334, 1983
3. Blomstedt P, Fytagoridis A, Åström M, Linder J, Forsgren L, Hariz MI: Unilateral caudal zona incerta deep brain stimulation for Parkinsonian tremor. Parkinsonism Relat Disord 18:1062–1066, 2012 Available: https://www.sciencedirect.com/science/article/pii/S1353802012002337.
Figures
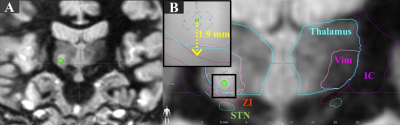
Figure 1. Figure 1. (A) Postoperative day 1 T2 MR Image showing the lesion located at ViM in the coronal plane. (B) Auto-segmented thalamus (cyan), ViM (pink), ZI (red), IC (purple), and STN (green) using Brainlab. The inserted black box shows a magnified view of distance measurement between target focus to ZI.
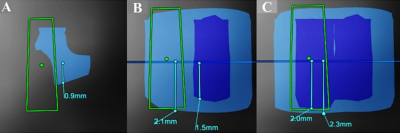
Figure 2. The representative image of accumulated thermal dose colored overlay extracted from the MRgFUS system. The distance was measured from focus to the inferior direction in the coronal plane after the 3rd (A), 4th (B), and the 5th sonication (C) at two thresholds: 17 CEM43 (light blue), and 240 CEM43 (dark blue).
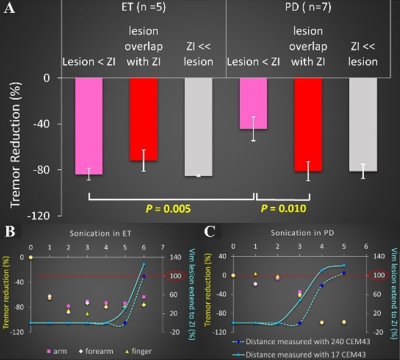
Figure 3. (A) Comparison of tremor reduction (%) between ET and PD depending on the lesion size increase from ViM towards ZI. Tremor reduction is measured when the lesion does not overlap with ZI (pink), when the lesion started to touch the ZI (red), when the lesion partially overlaps or expands beyond the ZI (grey). (B) Tremor reduction throughout the sonication course in ET. Significant tremor reduction was observed before the overlap (red-dotted line) in ET, but the overlap coincided with a significant drop of tremor amplitude in PD (C).