4037
Limbic Predominant Age-related TDP-43 Encephalopathy Neuropathological Change (LATE-NC) is Associated with Lower Diffusion Anisotropy1Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States
Synopsis
The MRI signature of limbic predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) has not been fully determined. In this work, we investigated the association of diffusion anisotropy with LATE-NC in autopsied brains of community-based older adults (N=148). Voxel-wise analysis revealed an independent association of lower fractional anisotropy (FA) with greater LATE-NC burden in medial temporal lobe white matter, controlling for other neuropathologies. The white matter connections implicated include fibers connecting cortical and subcortical regions where LATE-NC is typically found. Comparison of FA values across LATE-NC stages revealed that FA may allow detection of LATE-NC in later stages of the disease.
Introduction
Limbic predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is now well-recognized as a neurodegenerative disorder of the aging brain and has been associated with accelerated cognitive decline1,2. Most MRI studies of LATE-NC conducted to date have focused on brain morphometric abnormalities1–3, and the association of LATE-NC with other brain MRI characteristics remains largely undetermined. In this work, we hypothesized that abnormalities in white matter (WM) structural integrity caused by LATE-NC may be detected by means of diffusion tensor imaging (DTI). We first performed a voxel-wise analysis in autopsied brains from community-based older adults to map the spatial pattern of the association between ex-vivo fractional anisotropy (FA) and LATE-NC. We then identified the white matter connections traversing the regions implicated in LATE-NC according to the voxel-wise analysis. Finally, we investigated WM abnormalities at different LATE-NC stages and identified the earliest stage exhibiting significant FA anomalies.Methods
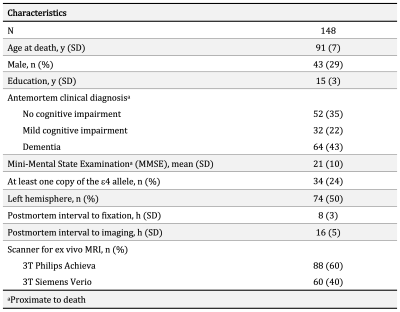
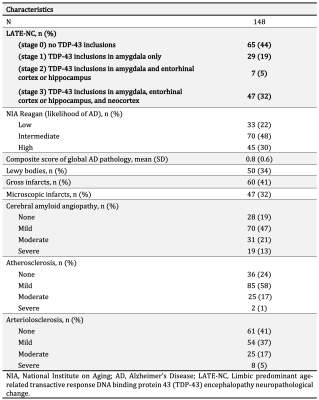
Participants, MRI, neuropathologyCommunity-based older adults (N=148) participating in two longitudinal cohort studies of aging4, the Rush Memory and Aging Project, and Religious Orders Study, were included in this work (Fig. 1). Cerebral hemispheres collected at autopsy were imaged with MRI ex-vivo within the first 24 hours after death. DTI data were collected on 3T clinical MRI scanners using a spin-echo echo planar diffusion imaging sequence with the following parameters: TE=105ms, TR=12.5s, voxel size = 2×2×2 mm3, b = 3,000 s/mm2 for 200 diffusion directions uniformly distributed in 3D space, and 72 b = 1,800 s/mm2 volumes. TORTOISE was used to correct eddy current distortions, calculate diffusion tensors, and generate FA maps5. The resulting FA maps were aligned to an ex-vivo FA template using ANTs registration6, and projected onto the corresponding white matter skeleton7. Following ex-vivo MRI, hemispheres underwent detailed histopathologic examination by a board-certified neuropathologist blinded to all data (Fig. 2). LATE-NC was summarized into 4 stages: stage 0 (no TDP-43 inclusions), stage 1 (TDP-43 inclusions in amygdala only), stage 2 (TDP-43 inclusions in amygdala and entorhinal cortex or hippocampus), stage 3 (TDP-43 inclusions in amygdala, entorhinal cortex or hippocampus, and neocortex)1.
Statistical analyses
Voxel-wise analysis was performed on the white matter skeleton to investigate the association of FA with LATE-NC, controlling for other neuropathologies (Alzheimer’s disease, Lewy bodies, cerebral amyloid angiopathy, gross and microscopic infarcts, atherosclerosis, arteriolosclerosis), demographics (age at death, sex, years of education), and other variables (postmortem interval to immersion in fixative, postmortem interval to ex-vivo MRI, total volume of white matter hyperintensities, scanner). Statistical analysis was performed using PALM (FMRIB, Oxford, UK) with 10,000 permutations and family-wise error rate (FWER) correction for multiple comparisons8. Statistical significance was set at p<0.05. The “regionconnect” tool of the IIT Human Brain Atlas (v.5.0) was used to extract probable connections passing through voxels showing significance in the voxel-wise analysis9. Lastly, FA values in regions of interest that showed significance in the voxel-wise analysis were compared between LATE-NC stages 1, 2, 3 and stage 0 (after adjustment for the aforementioned covariates). Since stage 2 had only few subjects (Fig. 2), stages 1 and 2 were grouped together.
Results
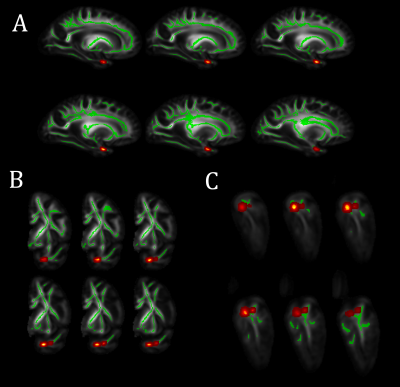
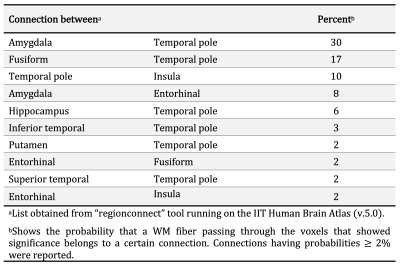
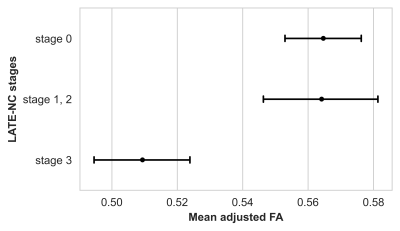
Voxel-wise analysis on the white matter skeleton revealed lower FA for greater LATE-NC burden in medial temporal lobe WM, controlling for other neuropathologies, demographics and covariates (Fig. 3). The connections traversing this WM region include fibers connecting the amygdala, temporal pole, hippocampus, entorhinal cortex, fusiform, insula, and putamen (Fig. 4). No voxel showed a positive association between FA and LATE-NC. Comparison of adjusted FA values in medial temporal lobe WM across LATE-NC stages revealed significant FA anomalies between stage 0 and 3 (ΔFA=-0.06, FDR-corrected p<10-6, Welch’s t-test) (Fig. 5). No significant difference in adjusted FA values was observed between stage 0 and stages 1 & 2 combined.Discussion
To our knowledge, this is the first study on the association of brain diffusion characteristics with LATE-NC. This investigation in autopsied brains from community-based older adults revealed an independent association of lower FA with greater LATE-NC burden in WM connecting cortical and subcortical regions where LATE-NC is typically found1,10,11 (Fig. 4). The lower FA for higher LATE-NC burden may reflect axonal damage associated with LATE-NC. The findings that FA values were significantly lower only in stage 3 indicate that FA may allow detection of LATE-NC in later stages of the disease. Future work will determine if FA is sensitive to stage 2 LATE-NC which had only few participants in this work and was therefore combined with stage 1. Although DTI data were acquired ex-vivo, we expect similar results in-vivo since we have previously shown that ex-vivo FA is linearly related to in-vivo FA for the same tissue preparation and imaging protocol used here12.Conclusion
The present study in autopsied brains of community-based older adults showed that lower FA is independently associated with greater LATE-NC burden, particularly in WM connections of a network of regions where LATE-NC is common. Our results also demonstrated that FA is sensitive to LATE-NC mainly in later stages of the disease. Overall, FA in medial temporal lobe white matter may potentially contribute towards the development of an in-vivo tool that combines multiple pieces of information to predict LATE-NC which, currently, can only be diagnosed at autopsy.Acknowledgements
National Institute of Neurological Disorders and Stroke (NINDS): UH2-UH3NS100599, UF1NS100599, R21NS076827
National Institute on Aging (NIA): R01AG064233, R01AG067482, R01AG017917, R01AG015819, RF1AG022018, R01AG056405, R01AG052200, P30AG010161, P30AG072975
References
- Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain. 2019;142(6):1503-1527.
- Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014;127(6):811-824.
- Bejanin A, Murray ME, Martin P, et al. Antemortem volume loss mirrors TDP-43 staging in older adults with non-frontotemporal lobar degeneration. Brain. 2019;142(11):3621-3635.
- Bennett DA, Buchman AS, Boyle PA, et al. Religious Orders Study and Rush Memory and Aging Project. J Alzheimer’s Dis. 2018;64(s1):S161-S189.
- Pierpaoli C, Walker L, Irfanoglu MO, et al. TORTOISE: an integrated software package for processing of diffusion MRI data. ISMRM 18th Annual Meeting, Stockholm, Sweden; 2010.
- Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033-2044.
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487-1505.
- Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage. 2014;92:381-397.
- Qi X, Arfanakis K. Regionconnect: Rapidly extracting standardized brain connectivity information in voxel-wise neuroimaging studies. Neuroimage. 2021;225.
- Josephs KA, Murray ME, Whitwell JL, et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol. 2016;131(4):571-585.
- Nag S, Yu L, Boyle PA, Leurgans SE, et al. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun. 2018;6(1):33.
- Yingjuan W, Dawe RJ, Evia AM, et al. Ex-Vivo Diffusion Anisotropy of Human Brain Hemispheres. ISMRM 24th Annual Meeting, Singapore; 2016.
Figures