4036
Gender-specific age-related changes in glymphatic function as measured by resting-state fMRI
Feng Han1, Yifan Yang1, and Xiao Liu1
1the Pennsylvania State University, State College, PA, United States
1the Pennsylvania State University, State College, PA, United States
Synopsis
The glymphatic system responsible for brain waste clearance may play an important role in aging and dementias. However, a lack of non-invasive tools for gauging glymphatic function in human subjects hindered the research on the age-related glymphatic changes. The global component of low-frequency (<0.1 Hz) resting-state fMRI signals was recently found coupled with CSF flow, and their coupling was used for quantifying the glymphatic function and found reduced significantly in neurodegenerative patients. Here we used the gBOLD-CSF coupling to evaluate glymphatic function change along healthy aging, and found it starts to decrease around age 55, particularly in females.
Introduction
Aging is associated with cognitive decline1. Although the mechanisms underlying this association remain elusive, neurological processes driving the brain waste clearance2 may play a role. Specifically, a glymphatic system that clears out brain toxins by CSF flow through the perivascular and interstitial spaces was found significantly impaired in aged mice as compared with younger ones3. However, the study of age-related changes in glymphatic system is largely absent in human subjects due to the lack of noninvasive tools for gauging the glymphatic function. Recently, the global component of low-frequency (<0.1 Hz) resting-state fMRI (rsfMRI) signals (gBOLD) was linked to the glymphatic function because of its larger amplitude during sleep4 and coupling to CSF low5. Consistent with this hypothesis, the coupling between the gBOLD and CSF signals was found decreased significantly in both Alzheimer’s disease6 and Parkinson’s disease with cognitive decline7. Moreover, the gBOLD-CSF coupling was correlated with age and gender in those aged clinical populations6. Here, we employed the gBOLD-CSF coupling measure to study the gender-specific age-related changes in glymphatic function using the Human Connectome Project Aging (HCP-A)8 dataset.Methods
Our study included 719 subjects (aged 36~100 years old) with four sessions of rsfMRI data from the HCP-A project. CSF fMRI signal changes originate from MR inflow effect5, and we extracted CSF signals from the bottom slice of fMRI acquisition to maximize this effect. Global rsfMRI BOLD (gBOLD) signal was obtained by averaging fMRI signals across all voxels within the brain9. The cross-correlation function was computed between the gBOLD and CSF signals for each fMRI session and then averaged across the 4 sessions for each subject. We then defined the gBOLD-CSF coupling as their correlation at the negative peak of +4 sec lag. We quantified the coupling changes with aging by grouping the entire cohort of subjects, the females, or the males, based on their ages (one group per 10 years) and then comparing the mean coupling measures across these age groups. We also separated the subjects into two groups with a cut-off age of 55 years old and then estimated the coupling-age association with a linear regression within each group. A two-sample t-test was used to evaluate the difference between neighboring groups (e.g., coupling for subjects aged 36~45 vs. subjects aged 46~55).Results
The averaged gBOLD-CSF cross-correlation function displayed a positive peak at -4-sec lag (r=0.38) whereas a negative one at the +4-sec lag (r=-0.32). (Fig. 1) This pattern is consistent with previous studies5–7. The gBOLD-CSF correlation at the 4-sec lag (negative peak) was used to quantify the gBOLD-CSF coupling. The gBOLD-CSF coupling showed significant changes across different age groups, and the changes are apparently nonlinear. The coupling metrics appeared stable before age 55, which is consistent with a lack of the coupling-age correlation within the younger (age<55) group (r=-0.02, p=0.74). The coupling measure decreased rapidly after age 55, leading to its significant (r=0.14, p<0.001; Fig. 2) correlation with age in the older (≥55) group. The gBOLD-CSF coupling is weaker in females compared to males (p<0.001, t-test; Fig. 3A), similar to the previous finding in AD-related groups6. More importantly, the age-related changes in gBOLD-CSF coupling showed different patterns across genders. The gBOLD-CSF coupling in the females showed a much steeper decline after 55, whereas the male group displayed a more gradual decrease (Fig. 3B).Discussions
We studied the gender-specific age-related changes in glymphatic function in human subjects using resting-state fMRI. The glymphatic function, as quantified by the gBOLD-CSF coupling strength, displayed a specific trajectory of changes with aging. It remains stable from age 36 to 55 and then starts to decrease around 55. While an overall similar age trajectory was observed for different gender groups, the decrease of the gBOLD-CSF coupling in females after ~55 years old is significantly larger and more abrupt than that in males. The age-related decrease in the glymphatic function appears 10-20 years earlier than the age-related increase in dementias10,11, and the gender-specific effect is similar in both, with females showing a larger and faster age-related cognitive change12. Overall, the gender-specific age-related glymphatic changes may represent an important aging process that might be linked to the development of dementias.Conclusion
We measured the brain glymphatic function using human rsfMRI signals through the coupling between the gBOLD and CSF signals. The glymphatic function showed an age-dependent decrease starting from ~55 years old and the decline is larger and more abrupt in the females than in the males.Acknowledgements
This work was supported by the National Institutes of Health (NIH) Pathway to Independence Award (K99/R00 5R00NS092996-03), the Brain Initiative award (1RF1MH123247-01), and the NIH R01 award (1R01NS113889-01A1).References
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, Penke L, Rafnsson SB, Starr JM: Age-associated cognitive decline 2009 doi:10.1093/bmb/ldp0332.
- Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J: The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019; 65:106–193.
- Kress BT, Iliff JJ, Xia M, Wang M, Wei Bs HS, Zeppenfeld D, Xie L, Hongyi Kang BS, Xu Q, Liew JA, Plog BA, Ding F, PhD RD, Nedergaard M: Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014; 76:845–614.
- Fukunaga M, Horovitz SG, Gelderen P van, Zwart JA de, Jansma JM, Ikonomidou VN, Chu R, Deckers RHR, Leopold DA, Duyn JH: Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging 2006 doi:10.1016/j.mri.2006.04.0185.
- Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, Lewis LD: Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science (80- ) 2019 doi:10.1126/science.aax54406.
- Han F, Chen J, Belkin-Rosen A, Gu Y, Luo L, Buxton OM, Liu X: Reduced coupling between cerebrospinal fluid flow and global brain activity is linked to Alzheimer disease–related pathology. PLoS Biol 2021; 19:1–257.
- Han F, Brown GL, Zhu Y, Belkin-Rosen AE, Lewis MM, Du G, Gu Y, Eslinger PJ, Mailman RB, Huang X, Liu X: Decoupling of Global Brain Activity and Cerebrospinal Fluid Flow in Parkinson’s Disease Cognitive Decline. Mov Disord 2021; 36:2066–768.
- Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Bookheimer SY, Brown TB, Buckner RL, Burgess GC, Coalson TS, Chappell MA, Dapretto M, Douaud G, Fischl B, Glasser MF, Greve DN, Hodge C, Jamison KW, Jbabdi S, Kandala S, Li X, Mair RW, Mangia S, Marcus D, Mascali D, Moeller S, Nichols TE, Robinson EC, Salat DH, et al.: Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage 2018; 183:972–849.
- Wong CW, Olafsson V, Tal O, Liu TT: The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage 2013 doi:10.1016/j.neuroimage.2013.07.05710.
- Alzheimer’s Association: 2021 Alzheimer’s disease facts and figures special report Race, Ethnicity and Alzheimer’s in America. Alzheimers Dement 2021; 17:327–40611.
- Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, Belle G Van, Jolley L, Larson EB: Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol 2002; 59:1737–4612.
- Levine DA, Gross AL, Briceño EM, Tilton N, Giordani BJ, Sussman JB, Hayward RA, Burke JF, Hingtgen S, Elkind MSV, Manly JJ, Gottesman RF, Gaskin DJ, Sidney S, Sacco RL, Tom SE, Wright CB, Yaffe K, Galecki AT: Sex Differences in Cognitive Decline among US Adults. JAMA Netw Open 2021; 4:1–13
Figures
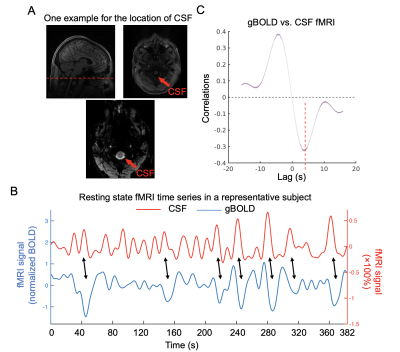
Fig. 1. gBOLD is coupled with CSF changes in HCP-A data. (A) Top left: location of bottom rsfMRI slice marked in corresponding structural MRI; top left&bottom: CSF region at bottom fMRI slice. (B) Coupled changes of global BOLD and CSF signal (black arrows) from an example. (C) Averaged gBOLD-CSF cross-correlation across 719 subjects. The red dashed line shows the time lag where the negative peak of the mean cross-correlation occurs. The shaded regions represent the area within one standard error of the mean.
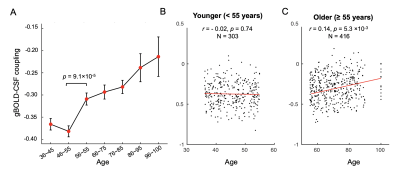
Fig. 2. The gBOLD-CSF coupling changes across ages. (A) Group-mean gBOLD-CSF coupling (red dots) decreased with aging but remained stable before 55 years old. A significant difference was also found between the neighboring groups aged around 55 (p = 9.1×10-5). Error bars represent the standard error of the mean. (B-C) The coupling-age correlation was significant among subjects older than 55 (linear regression; r = 0.14, p = 5.3×10-3; C), but not for the younger group (r = -0.02, p = 0.74; B).
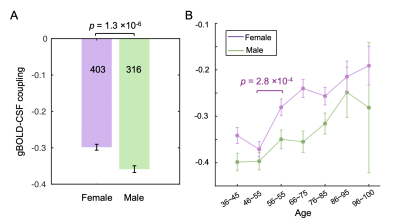
Fig. 3. Gender-specific changes of the gBOLD-CSF coupling with age. (A) The coupling strength was significantly weaker in the female (purple) group than the male (green) (p = 1.3×10-6, two-sample t-test). (B) The gBOLD-CSF coupling in the females showed a steep decline after 55, whereas the coupling strength in the male group decreased gradually. Error bars represent one standard error of the mean.
DOI: https://doi.org/10.58530/2022/4036