4033
Novel persistent tumor index to predict survival in pediatric high-grade gliomas treated with immunovirotherapy1Department of Diagnostic Imaging, St. Jude Children's Research Hospital, Memphis, TN, United States, 2Department of Biostatistics, St. Jude Children's Research Hospital, Memphis, TN, United States, 3Department of Pediatrics, University of Alabama at Birmingham, Birmingham, AL, United States, 4Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, AL, United States, 5Department of Radiation Oncology, University of Alabama at Birmingham, Birmingham, AL, United States, 6Department of Pathology, University of Alabama at Birmingham, Birmingham, AL, United States, 7Department of Neurosurgery, Brigham and Women's Hospital, Boston, MA, United States, 8Department of Neurosurgery, Boston Children's Hospital, Boston, MA, United States, 9Department of Neurosurgery, Harvard Medical School, Boston, MA, United States
Synopsis
Early indicators of the response to imunovirotherapy are currently limited due to the novelty of the therapies and unpredictability of responses. We propose using the persistent tumor index (PTI) derived from diffusion and perfusion MRI as a predictor of treatment response. In 10 high grade glioma patients treated with imunovirotherapy, only the baseline PTI measure was associated with progression free survival whereas other known imaging biomarkers of survival were not.
Introduction
Pediatric high-grade gliomas (pHGG) are rapidly fatal with median overall survival of only 5.6 months at recurrence after current standard therapy. New therapeutic approaches that improve survival are desperately needed. Of the many different approaches that are currently being tested, immunovirotherapy with oncolytic viruses has emerged as a promising strategy. However, response to immunovirotherapy is unpredictable, similar to other forms of immunotherapies. Currently, there is no quantifiable biomarker for predicting response to intratumoral immunovirotherapy, which makes treatment decisions challenging. We tested persistent tumor index (PTI), a novel imaging parameter based on both diffusion and perfusion imaging, for prediction of progression free survival (PFS), and we compared PTI with other known predictors of survival including tumor volume, median apparent diffusion coefficient (ADC), median cerebral blood volume (CBV), and fractional tumor burden (FTB).Methods
Patients: In a 3 x 3 prospective Phase 1 clinical trial, twelve children (7-18 years old) with progressive high-grade glioma were treated with genetically modified oncolytic G207 herpes simplex (oHSV) immunovirotherapy via intratumoral catheters in 3 or 4 loci.1 Six subjects received a 5 Gy dose of radiation within 24 hours of G207 to increase virus replication and spread. Ten subjects had enhancing tumor at the baseline scan and were included in this study. Five out of the included 10 subjects received the radiation.Image acquisition: Subjects were scanned on Phillips Achieva 3T magnet using a single imaging protocol. In addition to the anatomic imaging, diffusion weighted imaging (DWI) was obtained with b=0 & 1000 s/mm². Half of the gadobutrol (0.05mmol/kg) was injected as a pre-bolus. Dynamic susceptibility contrast (DSC) based perfusion imaging was obtained before, during and after injection of the remainder of the gadobutrol (0.05mmol/kg) at a rate of 4 mL/s.
Image analysis: Image analysis involved tumor segmentations, parametric map generation, registration of parametric maps and thresholding. We segmented enhancing components of each tumor using prior knowledge based automatic tumor segmentation method,2 manually verified tumor masks that included the enhancing components including the central necrotic area. ADC was calculated by the vendor provided software from the DWI raw data. Standardized CBV (stdCBV) was calculated from the DSC perfusion imaging by using IB Neuro™ software (Imaging Biometrics, Elm Grove, Wisconsin) and a synthetic CBV map (syCBV) was generated by dividing stdCBV map with 3575 to have the stdCBV map comparable to relative CBV map.3,4 The parametric maps (ADC, and syCBV) were co-registered with the tumor mask. Then, PTI was calculated using a homegrown Python-based thresholding pipeline by calculating the volumes of all the boxes of the tumor mask that had both ADC value of <1380 10-6 mm2/s and syCBV >1.5. Additionally, FTB4 was calculated using IB Rad Tech™ (Imaging Biometrics, Elm Grove, Wisconsin).
Statistical analysis: Cox regression analysis was performed for assessment of the association of the imaging parameters with PFS.
Results
PTI range for the 10 tumors was 0.1-10.96 cc, whereas volumes of the tumor mask ranged from 0.8-31.8 cc. ADC ranged from 842-1542 x 10-6 mm2/s, median stdCBV from 0.76-2.0, and FTB from 17.9% to 83.6%. Of the five imaging parameters tested, only baseline PTI was associated with PFS (HR=7.308; 95% CI-1.396, 38.26; p= 0.019).Discussion
We chose ADC value of <1380 10-6 mm2/s because Parger5 et al demonstrated median ADC of recurrent gliomas was of 1380 10-6 mm2/s and Hoxworth et al4 demonstrated syCBV of >1.2 could predict recurrent tumor with area under the receiver operator curve of 0.85. Although the small sample size is an inherent weakness of this data, the novel imaging biomarker PTI, at baseline, was associated with PFS in this exploratory study, whereas other known predictors of survival were not.Conclusion
Baseline PTI is associated with PFS and may be a useful biomarker for prediction of response to immunovirotherapy in pHGG. However, this parameter needs to be validated in a larger study before widespread use.Acknowledgements
The research was supported by grants from the U.S. Food and Drug Administration (R01FD005379), the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001417), Cannonball Kids cancer Foundation, the Rally Foundation for Childhood Cancer Research, Hyundai Hope on Wheels, St. Baldrick’s Foundation, the Department of Defense (W81XWH-15-1-0108), Andrew McDonough B+ Foundation, Kaul Pediatric Research Institute, and NIH/NCI Comprehensive Cancer Center Core Support Grant (P30 CA013148). In addition, the research was supported by the generosity of Kelsie’s Crew, Eli’s Block Party Childhood Cancer Foundation, the Eli Jackson Foundation, Jaxon’s F.R.O.G. Foundation, Battle for a Cure Foundation, Sandcastle Kids and St. Jude/ALSAC.References
1. Friedman GK, Johnston JM, Bag AK, et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N Engl J Med. Apr 29 2021;384(17):1613-1622. doi:10.1056/NEJMoa2024947
2. Zhang S, Edwards A, Wang S, Patay Z, Bag A, Scoggins MA. A Prior Knowledge Based Tumor and Tumoral Subregion Segmentation Tool for Pediatric Brain Tumors. 2021:arXiv:2109.14775. Accessed September 01, 2021. https://ui.adsabs.harvard.edu/abs/2021arXiv210914775Z
3. Prah MA, Al-Gizawiy MM, Mueller WM, et al. Spatial discrimination of glioblastoma and treatment effect with histologically-validated perfusion and diffusion magnetic resonance imaging metrics. J Neurooncol. Jan 2018;136(1):13-21. doi:10.1007/s11060-017-2617-3
4. Hoxworth JM, Eschbacher JM, Gonzales AC, et al. Performance of Standardized Relative CBV for Quantifying Regional Histologic Tumor Burden in Recurrent High-Grade Glioma: Comparison against Normalized Relative CBV Using Image-Localized Stereotactic Biopsies. AJNR Am J Neuroradiol. Mar 2020;41(3):408-415. doi:10.3174/ajnr.A6486
5. Prager AJ, Martinez N, Beal K, Omuro A, Zhang Z, Young RJ. Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol. May 2015;36(5):877-85. doi:10.3174/ajnr.A4218Figures
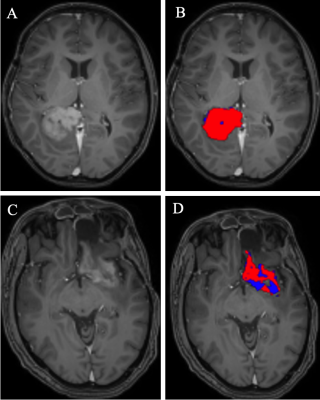
Figure 1. Baseline persistent tumor index (PTI) in subject #4 (A, B) who had PFS of 1.2 mo; and of subject #2 (C, D) who had PFS of >56.1 mo (no events as of this writing). Neither of these 2 subjects received additional low dose chemotherapy. A& C: post contrast T1-W images showing enhancing component of the tumor. B &D: PTI maps; red indicates thresholding of the tumor mask with ADC value of <1380 10-6 mm2/s and syCBV >1.5 suggestive of persistent tumor, whereas blue is ADC value of >1380 10-6 mm2/s and syCBV <1.5 suggestive of treatment effects.