4031
Mapping Brain Development in Infants and Young Children Using MPnRAGE T1 Relaxometry1Pediatrics, University of Wisconsin–Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin–Madison, Madison, WI, United States, 3Waisman Center, University of Wisconsin–Madison, Madison, WI, United States, 4Neuroscience Training Program, University of Wisconsin–Madison, Madison, WI, United States, 5Psychiatry, University of Wisconsin–Madison, Madison, WI, United States
Synopsis
Quantitative magnetic resonance imaging during the first years of life can provide novel insights into brain maturation, yet acquisition of these data are limited by challenges in imaging pediatric populations. Here, we assessed the self-navigated, motion robust MPnRAGE technique to acquire high-resolution T1-weighted structural brain images and quantitative T1 relaxometry maps in infants and young children. Our results demonstrate the ability to utilize MPnRAGE as a robust quantitative technique to assess early brain development.
Introduction
Human brain development is characterized by rapid, nonlinear growth throughout the first years of life1. Quantitative magnetic resonance imaging (qMRI) techniques offer unique opportunities to understand the dynamic patterns of emerging tissue microstructure that result from rapid neurodevelopmental changes, including reduction and compartmentalization of free water, changes in iron, and brain myelination2,3. However, many qMRI techniques are sensitive to motion, presenting significant challenges to obtaining high-quality and high-resolution data in pediatric populations. We recently developed a novel technique, termed MPnRAGE, that acquires multiple high-resolution volumetric magnetization-prepared rapid gradient echo (MPRAGE) images and allows for quantitative T1 (qT1) estimation4,5. MPnRAGE’s 3D radial acquisition further allows for self-navigated, retrospective motion correction5, and has been shown to generate high-quality T1-weighted (T1w) structural images and reproducible qT1 maps6,7, making MPnRAGE well-suited for investigations in pediatric populations. However, studies to date have been limited to older children, with little information stemming from work with infants and toddlers. Here, we present high-resolution T1w and qT1 MPnRAGE data acquired in a cohort of infants and children across the first 10 years of life. Age-related patterns of qT1 were measured to investigate the sensitivity of MPnRAGE qT1 estimates to the rapid neurodevelopmental changes across this time.Methods
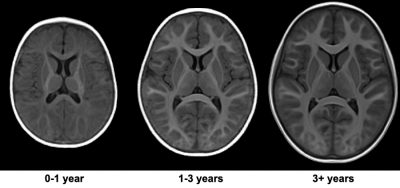
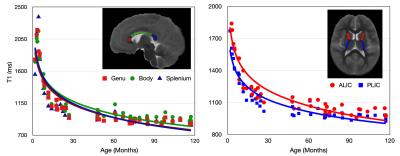
47 children (Mean Age: 51.4 months, age range: 2 months – 9.75 years years; 32 Male) were recruited and imaged using a 32-channel head RF array (Nova Medical, Wakefield, MA) on a 3T GE MR70 scanner. Children under 4 years of age were imaged during natural, non-sedated sleep, while children over 4 years of age were imaged awake while watching a movie or TV show. Whole-brain, high-resolution (1.0 mm isotropic) MPnRAGE images were acquired in the axial orientation. Acquisition parameters included TR = 4.9 ms, TE = 1.8 ms, and 386 views along the recovery curve, with excitation flip angles of 4°/8° for the first 304 views and 82 for the remaining views. A delay time of TD = 500 ms occurred after the last TR of each gradient-echo block to allow the signal to recover before the next preparation pulse. For children scanned during natural sleep, sinusoidal gradients and reduced gradient slew rates were used to reduce acoustic noise. The derated sinusoidal protocol had 192 and 64 views along the recovery curve with flip angles 4°/8° and TR = 7.7 ms and TE = 2.3 ms. Scan time was approximately 9 minutes for awake children and 14 minutes for sleeping infants. MPnRAGE T1w images were reconstructed using the entire k-space data set for each scan, with and without motion correction. A multi-pass fitting procedure was used to estimate parametric maps of T1, spin density (PD), B1, and the inversion efficiency8. qT1 maps were denoised using total variation minimization. Age and study-specific templates were created using MPnRAGE T1w images and ANTs9 (Fig. 1) and qT1 maps were transformed to template space using computed warps. ITK-Snap10 was used to manually delineate the corpus callosum (genu, body, splenium), and internal capsules (posterior and anterior limbs). Mean qT1 values were extracted, plotted against age, and fit to logarithmic models.Results
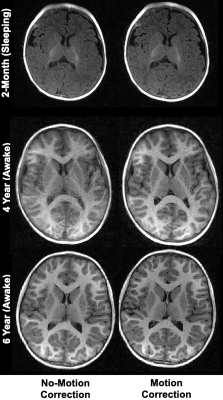
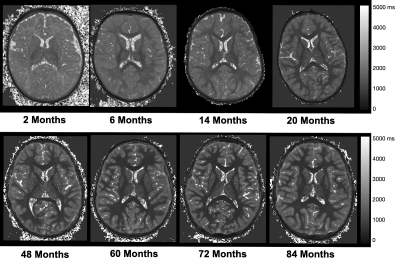
Representative axial slices of MPnRAGE T1w images before and after motion correction are shown in Fig. 2. These images highlight the robustness of the motion-correction algorithm to intra-scan motion, while also preserving images quality with minimal motion. As expected, qualitative changes in the gray/white matter contrast are apparent in the T1w images, with images becoming more “adult-like” with increasing age. Representative qT1 maps for children of different ages are shown in Fig 3. Alongside qualitative changes in T1w images, decreases of qT1 are observed across the brain, reflecting the progressive decreases in water content and brain myelination associated with brain maturation. Age-related trajectories from the corpus callosum and internal capsules reveal this developmental pattern follows a monotonically decreasing shape (Fig. 4). The overall spatiotemporal changes of qT1 are consistent with existing literature11, extending from deep to superficial brain regions in a posterior to anterior pattern.Discussion
In this work, we have examined the use of the MPnRAGE technique for obtaining structural brain images and quantitative T1 relaxometry maps in infancy and early childhood. We demonstrate high resolution and whole brain coverage, while the self-navigated, retrospective motion correction significantly improves image quality in the presence of intra-scan motion. Age-related patterns of qT1 were observed to decrease logarithmically, highlighting the rapid microstructural development during the first years of life. While measures were taken to reduce acoustic noise, these modifcations to image acquisition did increase the overall scan time. Future work will investigate alternative strategies for acoustic noise reduction as well as faster imaging. Moreover, as other quantitative relaxometry techniques have been used to study early neurodevelopment5,11, comparison with these other methods may also be informative.Conclusions
Our results demonstrate that MPnRAGE provides high-resolution, motion-robust T1w and quantitative T1 relaxometry in infants and young children. This technique may help advance the use of quantitative imaging in pediatric and other challenging populations. Moreover, given the sensitivity of T1 relaxation to neurodevelopmental changes in the brain, this technique may be informative for characterizing patterns of early brain development as well as assessing relationships of emerging cognition or specific risk factors of early neurodevelopment.Acknowledgements
We sincerely thank the children and families who participated in this research. This work was supported by grants R34 DA050258 (Dr. Alexander) from the National Institute of Drug Abuse; R00 MH11056 (Dr. Dean) from the National Institute of Mental Health, National Institutes of Health. Infrastructure support was also provided, in part, by grant U54 HD090256 from the Eunice Kennedy Shriver NICHD, National Institutes of Health (Waisman Center).References
1 Giedd, J. N. & Rapoport, J. L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67, 728-734, doi:10.1016/j.neuron.2010.08.040 (2010).
2 Deoni, S. C. Quantitative relaxometry of the brain. Top Magn Reson Imaging 21, 101-113, doi:10.1097/RMR.0b013e31821e56d8 (2010).
3 Lebel, C. & Deoni, S. The development of brain white matter microstructure. Neuroimage 182, 207-218, doi:10.1016/j.neuroimage.2017.12.097 (2018).
4 Kecskemeti, S. et al. MPnRAGE: A technique to simultaneously acquire hundreds of differently contrasted MPRAGE images with applications to quantitative T1 mapping. Magn Reson Med 75, 1040-1053, doi:10.1002/mrm.25674 (2016).
5 Kecskemeti, S. et al. Robust Motion Correction Strategy for Structural MRI in Unsedated Children Demonstrated with Three-dimensional Radial MPnRAGE. Radiology 289, 509-516, doi:10.1148/radiol.2018180180 (2018).
6 Kecskemeti, S. & Alexander, A. L. Three-dimensional motion-corrected T1 relaxometry with MPnRAGE. Magnetic Resonance in Medicine 84, 2400-2411, doi:https://doi.org/10.1002/mrm.28283 (2020).
7 Kecskemeti, S. R. & Alexander, A. L. Test-retest of automated segmentation with different motion correction strategies: A comparison of prospective versus retrospective methods. NeuroImage 209, 116494, doi:https://doi.org/10.1016/j.neuroimage.2019.116494 (2020).
8 Kecskemeti, S., Freeman, A., Travers, B. G. & Alexander, A. L. FreeSurfer based cortical mapping and T1-relaxometry with MPnRAGE: Test-retest reliability with and without retrospective motion correction. NeuroImage242, 118447, doi:https://doi.org/10.1016/j.neuroimage.2021.118447 (2021).
9 Tustison, N. J. et al. The ANTsX ecosystem for quantitative biological and medical imaging. Scientific Reports 11, 9068, doi:10.1038/s41598-021-87564-6 (2021).
10 Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116-1128, doi:10.1016/j.neuroimage.2006.01.015 (2006).
11 Deoni, S. C., Dean, D. C., 3rd, O'Muircheartaigh, J., Dirks, H. & Jerskey, B. A. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage63, 1038-1053, doi:10.1016/j.neuroimage.2012.07.037 (2012).
Figures