4030
Deep Learning Model for Automatic Fetal Brain Landmark Localization in MRI
Jie Deng1,2, Xuchu Liu2, Jubril O Adepoju2, and Sharon E Byrd2
1Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, United States, 2Diagnostic Radiology and Nuclear Medicine, Rush University Medical Center, Chicago, IL, United States
1Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, United States, 2Diagnostic Radiology and Nuclear Medicine, Rush University Medical Center, Chicago, IL, United States
Synopsis
Fetal MRI provides great anatomic details for diagnosis of perinatal disorders when ultrasound is inconclusive in assessing fetal anatomy during all phases of gestation. Immediate interventions can be attempted when abnormal anatomic changes of fetal brain structures are identified early during gestation. Size measurements of a fetal brain structure can be realized by identifying several landmarks of the structure accurately and calculating the distance between the landmarks. We developed a deep learning model that exploits U-net as a ‘transforming’ function to learn imaging features adjacent to a landmark point and predict the landmark location automatically.
INTRODUCTION
Fetal MRI provides clear images for diagnosis and early detection of perinatal disorders of development. MRI is often considered if ultrasound is inconclusive when assessing fetal anatomy during all phases of gestation. Several life-changing pathologies are usually defined by abnormal anatomic measurements of certain developing fetal brain structures [1]. MRI acquisitions on fetus are challenging due to body habitus of the mother, fluid in uterus, small fetal anatomy, and fetus movement. Single-shot sequences are acquired multiple times at different imaging planes in hope of catching the ‘motion-freeze’ images that provide sufficient spatial resolution and anatomical details. Accurate measurement of fetal brain anatomy is essential for differentiating normal structures from hypoplastic, absent, or malformed brain structures at different gestational ages of the fetuses, which can be highly variable. Fetal MRI interpretation can be time-consuming and radiologist experience dependent. In this study, we developed a novel deep learning model that exploits U-net as a ‘transforming’ function to learn imaging features adjacent to a landmark point and predict the landmark location [2-4]. The current work focuses on identifying landmarks on pons and vermis.METHODS
Landmarks of vermis and pons were drawn by a radiologist as the ground truth on the selected sagittal HASTE images. A rotationally symmetric gray-scale image mask with Gaussian distribution function f(x) = exp(-4πr2/R2), r = [0, R], where R was the radius of a circle centered on the annotated landmark point (Fig. 1), was used as model output in training process. R was proportional to the fronto-occipital diameter of the fetal brain and can be optimized during model training. A U-net model [5] was trained to transform an input image to a gray-scale mask with its center of gravity representing the location of the predicted landmark point (Fig. 2). Adaptive moment estimation (Adam) was used to minimize the mean square error between the ground truth gaussian distribution mask and predicted mask centered on the landmark point, with number of epochs = 100, batch size = 20. In validation and testing, if the predicted landmark position was within 10 pixels radius of the annotated landmark position, it was considered as an accurate prediction (Fig.3). In addition, a rotational symmetry (δ) parameter of the predicted mask was calculated as the predication error, defined as the separation between the centers of gravity by setting the thresholding above 0.8 and above 0.1 on the predicted mask image (Fig. 3). A smaller δ indicated more symmetric mask closer to the Gaussian distribution function and thus better prediction outcome. A total of 90 sagittal HASTE fetal brain series with diagnostic image quality were selected, with 66.7% (60/90) for training, 16.7% (15/90) for validation, and 16.7% (15/90) for testing. All images were resized to 512×512 and augmented by flipping, turning, and rotating to expand the dataset by 12 times.RESULTS
Six models were trained separately to identify 6 landmarks (2 for pons and 4 for vermis), and four-fold cross-validations were performed to calculate the accuracy in testing dataset. The highest accuracy of 96% and 97% for anterior/posterior (A/P) pons landmarks, 98% and 83% for A/P vermis landmarks, 91% and 92% for superior/inferior (S/I) vermis landmarks were achieved (Table 1). Nonetheless, different folds of testing dataset resulted in variable accuracies, mainly due to various image quality and the size of brain anatomy at different gestational ages of the fetuses. Furthermore, we created an interactive tool to help radiologist identify the landmarks of pons and vermis on fetal MRI more efficiently (Fig. 4). For each patient, multiple MRI series were uploaded to a server and all sagittal images were processed using the U-net model, from which an image with the best prediction outcome (i.e. smallest δ) was automatically selected for radiologist’s second check. On the user interface, a radiologist can manually adjust each landmark position if the predicted location is not satisfactory. After all landmark locations are confirmed by the radiologist, the distance between two related landmarks on a brain structure can be calculated and compared with the published gestational age based fetal brain anatomy measurements to determine if the structure is normal or abnormal.DISCUSSION
We developed a novel method for identifying anatomic landmarks on fetal brains and demonstrated promising prediction accuracy on the pons and vermis structures. The method also generated a prediction error parameter δ that informs the radiologist of the confidence level in model prediction for each landmark. The main limitation of this study was small sample size with only 60 fetal brain series for training. In addition, the size of brain structure and tissue contrast vary a lot in fast developing fetal brains, which makes learning imaging features very challenging. Finally, we established a pipeline consisting of a deep learning prediction model followed by an interactive second check tool to facilitate radiologist in locating, confirming, or adjusting the landmarks on the best selected image slice more efficiently. Future work includes collection of more normal and abnormal fetal MRI at different gestational phases to further improve the model.CONCLUSION
Fetal brain anatomic measurements on MRI can be achieved by using a deep learning model to predict critical landmarks automatically followed by a quick second check by the radiologists.Acknowledgements
No acknowledgement found.References
[1] Glenn OA, Barkovich AJ. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis, part 1. AJNR Am J Neuroradiol. 2006 Sep;27(8):1604-11. PMID: 16971596.[2] Liu C, et al. Misshapen Pelvis Landmark Detection With Local-Global Feature Learning for Diagnosing Developmental Dysplasia of the Hip. IEEE Trans Med Imaging. 2020 Dec;39(12):3944-3954. doi: 10.1109/TMI.2020.3008382. Epub 2020 Nov 30. PMID: 32746137.[3] Julia M. H, et al. Deep Learning-Based Regression and Classification for Automatic Landmark Localization in Medical Images. arXiv:2007.05295v1[4] Ebner M, et al. An automated framework for localization, segmentation and super-resolution reconstruction of fetal brain MRI. Neuroimage. 2020 Feb 1;206:116324. doi: 10.1016/j.neuroimage.2019.116324. Epub 2019 Nov 6. PMID: 31704293; PMCID: PMC7103783.[5] Zhou Z, et al. UNet++: A Nested U-Net Architecture for Medical Image Segmentation. Deep Learn Med Image Anal Multimodal Learn Clin Decis Support (2018). 2018 Sep;11045:3-11. doi: 10.1007/978-3-030-00889-5_1. Epub 2018 Sep 20. PMID: 32613207; PMCID: PMC7329239.Figures
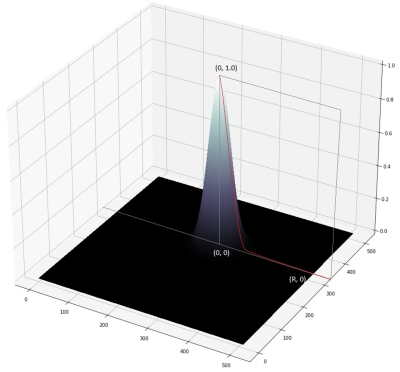
Figure 1. Rotationally symmetric Gaussian distribution function with a radius of R, and its center of gravity represent the position of landmark point.
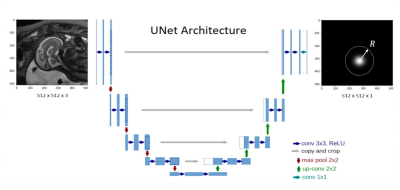
Figure 2. The structure of a U-net model transforming a sagittal fetal brain image to a gray-scale mask with its center of gravity representing the location of the landmark point. The radius R of the Gaussian distribution function is a hyper-parameter optimized during training.
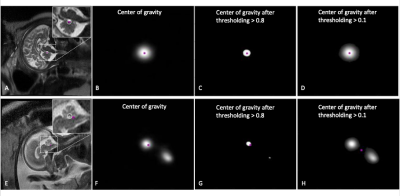
Figure 3. Examples of model predicted landmark with prediction error evaluation. In an accurate case (A-D), the predicted landmark position (purple point), calculated as the center of gravity of the predicated gray-scale mask image (B), falls within 10 pixels radius (white circle in A) of the annotated landmark (white point in A). The center of gravity above threshold of 0.8 and 0.1 matches well (C, D), indicating small prediction error. In another case (E-H), the center of gravity from the mask image above threshold of 0.8 and 0.1 are separated, indicating a large prediction error.
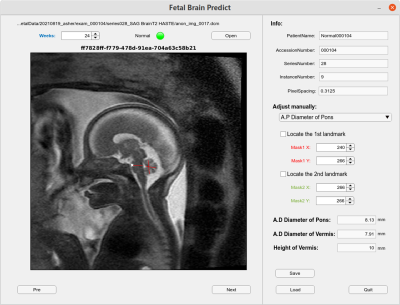
Figure
4. Web-based
interactive tool integrating deep learning prediction of landmarks is created
for radiologist to quickly locate, confirm, or adjust the landmarks (white
cross point) on the best selected image slice. After the landmark locations are
confirmed, the distance (red lines) between two related landmarks of a certain
brain structure is calculated and compared with the published data to determine
if the structure development at certain gestational age is normal or abnormal.
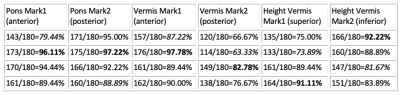
Table
1. The
accuracy in model prediction of each landmark of pons and vermis in testing
dataset in four-fold cross validations, and the highest accuracies are
highlighted.
DOI: https://doi.org/10.58530/2022/4030