4029
Sex-differences in resting-state brain activity in children with concussion in comparison to matched healthy controls.1Department of Electrical and Computer Engineering, McMaster University, Hamilton, ON, Canada, 2Child Health and Exercise Medicine Program, Department of Pediatrics, McMaster University, Hamilton, ON, Canada, 3Imaging Research Centre, St. Joseph's Healthcare, Hamilton, ON, Canada, 4School of Rehabilitation Science, McMaster University, Hamilton, ON, Canada, 5CanChild Centre for Childhood Disability Research, McMaster University, Hamilton, ON, Canada, 6School of Biomedical Engineering, McMaster University, Hamilton, ON, Canada, 7Department of Radiology, McMaster University, Hamilton, ON, Canada
Synopsis
There are known sex-differences with respect to the clinical presentation of pediatric concussion, with girls reporting more symptoms and symptoms with greater severity than boys. This is the first study to show that there are also sex-differences in resting state brain activity in children with concussion, suggesting that the sex-specific clinical presentation may have neurological underpinnings. Specifically, girls with concussion had resting state disturbances that were not present in boys, which is consistent with the variable symptom presentation of the injury. Continued research into these resting state differences is encouraged.
Introduction
The incidence of pediatric concussion is rising, with a marked increase in injury incidence in recent decades1,2. Accordingly, research activity into pediatric concussion has also peaked during this period, and a growing evidence base now suggests that there are marked sex-differences with respect to the clinical presentation of pediatric concussion3-5. Of note, girls with concussion report more symptoms and symptoms with greater severity than boys6, and research also suggests that long-term outcome also varies by sex7-9. More specifically, two large-scale cohort studies suggest that girls are at greater risk of protracted symptom recovery than boys with concussion4,9. However, to date, no studies have examined whether there are sex-differences with respect to the primary neuropathology of concussion (i.e., resting state functional brain activity disturbances). Data on adults with concussion suggests that there may be sex-specific variability with respect to resting state brain activity10. Therefore, this led us to explore, for the first time, whether there are also sex-differences with respect to resting state functional brain activity in children with concussion.Methods
Children with concussion (n=27; mean 11.2 ± 6.2 years old, 55.6% female) were recruited from the emergency department of a large, urban hospital with a large catchment area. Concussion diagnosis was confirmed by an emergency department physician with experience in concussion and sports medicine based on a neurological, vestibular, and physical exam. Control data (matched 1:1) were retrieved from the open-source ABIDE-II database, matched to participants on age and sex. All children were imaged, on average, within the first month of their injury (mean 28.8 ± 14.5 days). Imaging was performed using a 3-Tesla GE Discovery MR750 scanner with a 32-channel phased array head receive coil. For MRI data collection, a 3-plane localizer with calibration sequences was first acquired. Anatomical images were then collected using a 3D inversion recovery-prepped fast SPGR T1-weighted sequence (TE/TR/TI=4.25/11.36/450ms, flip angle=12°, 512x256 matrix interpolated to 512x512, 22cm axial FOV, 0.43mm in-plane, 1mm thick). Resting state imaging was done using a single shot gradient echo EPI sequence (TR/TE=2000/35ms, flip angle=90°, 64x64 matrix, 180 time points, 22cm FOV, 3.44mm in-plane 3mm thick), with participants asked to keep their eyes open not think of anything in particular. B0 maps were acquired for resting state scans, using the same geometric prescription as the resting state scan. Participant data were unwarped using the epiunwarp script11, and the remainder of pre-processing was performed in CONN 19c12 (which draws on some the functionality of SPM1213), run on MATLAB R2020a. More specifically, the pre-processing pipeline involved: 1) Functional realignment14, 2) Slice-timing correction15, 3) Functional data outlier detection using SPM’s Artifact Detection Tool (ART)13, 4) Direct segmentation and normalization/registration of functional data to MNI space (1mm and 2mm isotropic voxels for anatomical and functional data, respectively), based on posterior tissue probability maps, and 5) spatial smoothing of functional data with a Gaussian kernel of full-width at half-maximum of 6mm. Next, de-noising was performed in CONN which involved 1) ordinary least squares regression to project out noise components (associated with cerebral white matter and cerebrospinal regions16, outlier scans17, and subject motion18) using CONN’s anatomical component-based noise correction procedure (aCompCorr), and 2) temporal filtering (0.008Hz and above 0.01Hz). Seed-based and ROI-to-ROI connectivity measures were then computed, with seeds from four, large-scale, validated and clinically-salient (in pediatric concussion and otherwise) resting-state brain networks19-22: default mode network (DMN, seeded at the posterior cingulate cortex [1, -61, 38]), salience network (SN, seeded at the anterior cingulate cortex [0, 22, 35]), fronto-parietal network (FPN, seeded at the lateral pre-frontal cortices), and sensorimotor network (SMN, seeded superiorly at the pre-central gyrus [0, -31, 67]). Cluster-level inferences were made per Threshold Free Cluster Enhancement (TFCE)23 (with 10,000 permutations), and between groups contrasts were performed using independent samples t-tests, with family-wise error corrected p-values.Results
In females with concussion vs. healthy females, seed-based analyses showed hypo-connectivity between the anterior cingulate cortex of the SN and the precuneus (TFCE=1173.6, p=FWE=0.002) and cingulate gyrus (TFCE=1039.7, p-FWE=0.008), and the posterior cingulate cortex of the DMN and the paracingulate gyrus (PCC) (TFCE=870.1, p-FWE=0.015) and sub-callosal cortex (TFCE=795.4, p-FWE=0.037). Further, hyper-connectivity was observed between the lateral pre-frontal cortex and inferior frontal gyrus (TFCE=1215.4, p-FWE=0.002) and lateral occipital cortex (TFCE=854.9, p-FWE=0.020) and between the PCC and cerebellum (TFCE=791.0, p-FWE=0.038) (Figures 1 & 2). ROI analyses showed primarily patterns of hyper-connectivity in females (Figure 3). No differences were observed between males with concussion and healthy males on seed-based or ROI analyses.Discussion
This is the first study to show that there are sex-differences in resting state brain activity in children with concussion, which may explain the sex-specific variability in the clinical presentation of the injury. These data are consistent with what has been observed in adults10. Further research is needed to understand the clinical correlates of sex-specific resting state functional brain disturbances and salient clinical outcomes.Conclusion
This is the first study to show that there are sex-differences with respect to resting state functional brain activity in pediatric concussion. This has implications for sex-specific management of pediatric concussion.Acknowledgements
This study was supported by the Canadian Institutes of Health Research (MOP# 31257) and Doctoral Funding to B Sharma through the Canadian Institutes of Health Research (CIHR-CGS-D #157864). BW Timmons is the Canada Research Chair in Child Health & Exercise Medicine. We thank the participants for their time and effort.
References
1. Zhang AL, Sing DC, Rugg CM, et al. The rise of concussions in the adolescent population. Orthopaedic journal of sports medicine. 2016;4(8):2325967116662458.
2. Zemek RL, Grool AM, Rodriguez Duque D, et al. Annual and Seasonal Trends in Ambulatory Visits for Pediatric Concussion in Ontario between 2003 and 2013. J Pediatr. 2016.
3. Koerte IK, Schultz V, Sydnor VJ, et al. Sex‐Related Differences in the Effects of Sports‐Related Concussion: A Review. Journal of neuroimaging. 2020;30(4):387-409.
4. Levin HS, Temkin NR, Barber J, et al. Association of Sex and Age With Mild Traumatic Brain Injury–Related Symptoms: A TRACK-TBI Study. JAMA Network Open. 2021;4(4):e213046-e213046.
5. Merritt VC, Padgett CR, Jak AJ. A systematic review of sex differences in concussion outcome: What do we know? The Clinical Neuropsychologist. 2019;33(6):1016-1043.
6. Alsalaheen B, Almeida A, Eckner J, et al. Do male and female adolescents report symptoms differently after concussion? Brain injury. 2021:1-7.
7. Barlow KM. Postconcussion syndrome: a review. Journal of child neurology. 2016;31(1):57-67.
8. Clair R, Levin Allen S, Goodman A, et al. Gender differences in quality of life and symptom expression during recovery from concussion. Applied Neuropsychology: Child. 2020;9(3):206-214.
9. Ledoux A-A, Tang K, Yeates KO, et al. Natural progression of symptom change and recovery from concussion in a pediatric population. JAMA pediatrics. 2019;173(1):e183820-e183820.
10. Shafi R, Crawley AP, Tartaglia MC, et al. Sex-specific differences in resting-state functional connectivity of large-scale networks in postconcussion syndrome. Scientific Reports. 2020;10(1):1-12.
11. Davis AD, Noseworthy MD. Motion and distortion correction of skeletal muscle echo planar images. Magnetic resonance imaging. 2016;34(6):832-838.
12. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2(3):125-141.
13. Ashburner J, Barnes G, Chen C-C, et al. SPM12 manual. Wellcome Trust Centre for Neuroimaging, London, UK. 2014;2464.
14. Andersson JL, Hutton C, Ashburner J, et al. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13(5):903-919.
15. Henson R, Buechel C, Josephs O, et al. The slice-timing problem in event-related fMRI. NeuroImage. 1999;9:125-.
16. Behzadi Y, Restom K, Liau J, et al. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90-101.
17. Power JD, Mitra A, Laumann TO, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320-341.
18. Friston KJ, Williams S, Howard R, et al. Movement‐related effects in fMRI time‐series. Magnetic resonance in medicine. 1996;35(3):346-355.
19. Van Den Heuvel MP, Pol HEH. Exploring the brain network: a review on resting-state fMRI functional connectivity. European neuropsychopharmacology. 2010;20(8):519-534.
20. Smitha K, Akhil Raja K, Arun K, et al. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. The neuroradiology journal. 2017;30(4):305-317.
21. Seitzman BA, Snyder AZ, Leuthardt EC, et al. The state of resting state networks. Topics in Magnetic Resonance Imaging. 2019;28(4):189-196.
22. Chamard E, Lichtenstein JD. A systematic review of neuroimaging findings in children and adolescents with sports-related concussion. Brain injury. 2018;32(7):816-831.
23. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83-98.
Figures
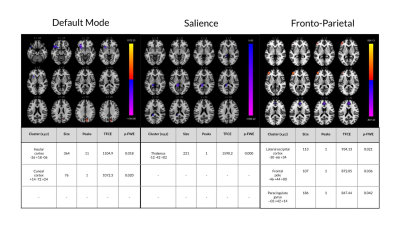
Figure 1: rs-fMRI differences between females with concussion and healthy age-matched females. Clusters (x, y, z) denote standard MNI coordinates at the center of cluster mass, and size represents number of voxels. Only statistics for clusters that survived a p-FWE (family-wise error) <0.05 per TFCE are displayed.
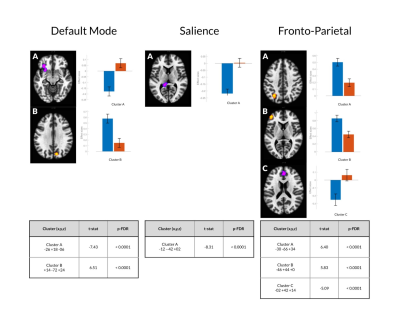
Figure 2. For each significant cluster (per threshold-free cluster enhancement; TFCE), the effect size associated with that cluster by group (healthy females vs. females with concussion) is depicted below. An associated independent samples t-test (with 25 degrees of freedom) compares the effect sizes at each cluster between groups. Blue and red bars indicate effect sizes for the concussion and control cohorts, respectively.
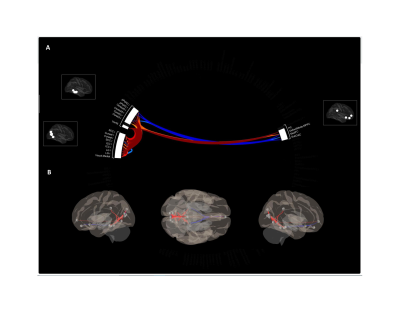
Figure 3. (A) Significantly increased (warm colours) and decreased (cool colours) ROI-to-ROI connectivity in females with concussion in comparison to healthy female controls. (B) Depiction of the ROI-to-ROI changes are summarized in panel A on a 3D glass brain to more clearly depict altered patterns of connectivity in an anatomically relevant space.