4026
Disrupted structural-functional coupling in children with prenatal alcohol exposure1Victorian Infant Brain Study (VIBeS), Murdoch Children's Research Institute, Melbourne, Australia, 2Florey Department of Neuroscience and Mental Health, University of Melbourne, Melbourne, Australia, 3Institute for Radiological Research, Chang Gung University and Chang Gung Memorial Hospital, Taoyuan, Taiwan, 4Department of Psychiatry, Chang Gung Memorial Hospital, Taoyuan, Taiwan, 5Turner Institute for Brain and Mental Health, Monash University, Clayton, Australia
Synopsis
In this study, we investigated whether prenatal alcohol exposure (PAE) disrupts the coupling strength between SC and static FC or dynamic FC using multimodal connectomes. Greater coupling strengths were identified in the PAE T1 and PAE T1-T3 groups within males, indicative of brain structural and functional changes in children with PAE. Distinct age effects on coupling strength were also revealed. Further, the reduction of assortative coefficients indicated that PAE could compromise the robustness of brain networks. These findings suggest that multimodal connectomes, especially integrating network dynamics, could be advantageous to revealing brain structural and functional changes.
INTRODUCTION
Accumulating evidence has shown that children with prenatal alcohol exposures (PAE) face high risks of brain abnormalities1. During the last decade, structural and functional connectivity (SC-FC) coupling has emerged as a powerful noninvasive approach for quantifying brain networks2, providing a quantitative method for simultaneously characterizing brain organization disruptions both functionally and structurally. Previous studies have investigated the PAE effects on children’s brain organization using either FC or SC independently. The integration of SC and FC for capturing potential unique and complementary information such as via SC-FC coupling has not yet been explored within this area. In this study, we aimed to investigate whether PAE disrupts the coupling strength between SC and static FC (SC-SFC) or dynamic FC3 (SC-DFC).METHODS
Subjects: Ninety subjects participated this study, including 24 children with no PAE (nPAE) (M/F: 15/9, age: 7.30±0.41), 27 subjects with PAE only in trimester 1 (PAE T1) (M/F: 8/19, age:7.30±0.34), and 39 subjects with PAE throughout pregnancy (PAE T1-3) (M/F: 22/17, age: 7.27±0.21).Data acquisition: Diffusion-weighted images (DWIs) were collected on 3T Siemens Prisma with 4 b-value (b=0/750/2000/2800 s/mm2 at 22/25/45/60 directions, respectively), TR/TE=3500/67ms, flip-angle=90°,voxel size: 2.02×2.02×2mm3, partial Fourier factor = 0.75, multiband factor=2. Resting-state fMRI (rs-fMRI): TR/TE = 1500/33ms, flip-angle=85°, voxel size=2.45×2.45×2.5 mm3 , multiband factor=3, total time points = 206. Anatomical T1-weighted images were acquired using the 3D MPRAGE sequence (TR/TE/TI: 2550/7.5/1550ms).
Data analysis: DWIs were preprocessed with denoising, distortion and motion correction4, and B1 bias field correction. FODs were estimated with multi-shell multi-tissue constrained spherical deconvolution5. Quantitative tractography was performed using MRtrix3’s6 ACT7 and SIFT28. Structural connectomes were constructed based on the AAL atlas with 116 brain regions.Rs-fMRI data were preprocessed as follows: motion correction, confound regression, brain extract and normalization, band filtering, and volume censoring9. AAL parcellation was employed to extract region-wise time series. Functional connectivity was estimated using Pearson correlation coefficient.
Coupling strength between SC and FC: Coupling strengths of SC-SFC/DFC were calculated at individual level. For DFC, 5 fMRI data segments were employed, with each segment being composed of 20 time points. One-hundred time points were employed to compute static FC for each subject. Whole-brain coupling strength was defined as the Pearson correlation coefficient between log-transformed whole-brain SC and corresponding SFC/DFC.
Assortative coefficients calculation: The edge weights of structural network were normalized to the range of [0,1], which was then employed to generate the supra-adjacency matrix along with the static functional network.Assortative coefficient was defined as the Pearson correlation coefficient of weights between linked nodes. Subsequently, the assortative coefficient was computed from the generated supra-adjacency matrix at individual level.
Statistical analyses
(1) ANCOVA analysis: SC-SFC coupling strengths, as well as the assortative coefficients, were compared between the PAE groups using ANCOVA, controlling for age and gender.
(2) Linear mixed-effects (LME) model analysis: Five DFC networks, representing 5 repeated measures for each subject, were employed for computing the repeated measures of coupling strength for SC-DFC. Subsequently, the repeated measures of coupling strength of SC-DFC were analyzed using the LME model. Specifically, the analyses were performed by considering the effects of gender and age separately.
RESULTS
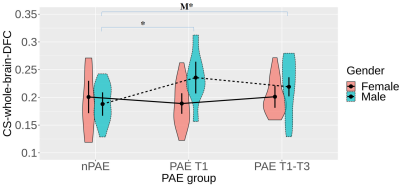
No significant differences were detected between the PAE groups (nPAE, PAE T1, PAE T1-T3) with the LME analysis after controlling for age and gender.Figure 1 reveals that significant differences were only observed among the male groups. Specifically, within the males, the PAE T1 group had significantly greater coupling strengths than the nPAE group, whereas only marginally significant difference was detected from the PAE T1-T3 group.
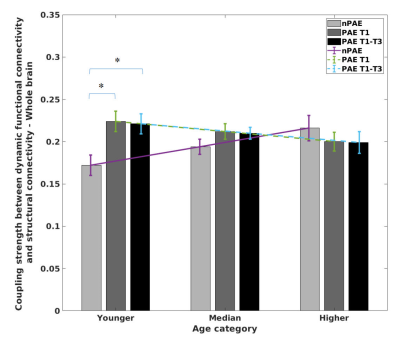
Figure 2 shows the results of whole-brain SC-DFC coupling across the three age ranges using the LME model. Within the younger ages, significantly greater coupling strengths were identified in the PAE T1and tPAE T1-T3 groups compared with the nPAE group. No significant PAE group differences were observed for the median or higher ages.
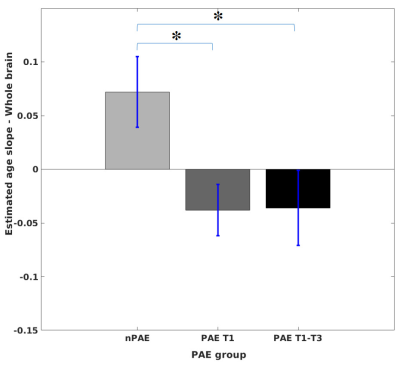
Figure 3 demonstrates that significant decreases in age slopes were identified in the PAE T1 and the PAE T1-T3 groups compared to the nPAE group.
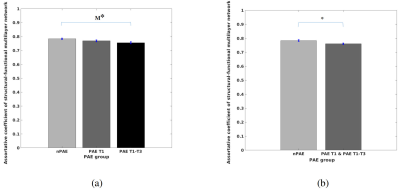
Figure 4a shows that the PAE T1-T3group has lower assortative coefficients than the nPAE group, but this was only marginally significant. No significant differences were detected between any other groups. A combined PAE T1 and T1-T3 group yielded significantly lower assortative coefficients than the nPAE group (Figure 4b).
DISCUSSION
Greater coupling strengths were identified in the PAE T1 and PAE T1-T3 groups within males, indicative of brain structural and functional changes. Consistent with literature10, the present findings suggest that PAE may have differential effects according to gender. Greater coupling strengths were also identified in the PAE T1 and PAE T1-T3 groups in the younger age group, suggesting that the PAE effect may be modulated by age. The slope differences between the PAE and the nPAE groups suggest significant interaction effects (PAE×Age). Further, the reduction of assortative coefficients indicated that PAE could compromise the robustness of brain networks. These findings suggest that multimodal connectomes, especially integrating network dynamics, could be advantageous to revealing brain structural and functional changes.Acknowledgements
No acknowledgement found.References
1.Lees, B., et al., Association of Prenatal Alcohol Exposure With Psychological, Behavioral, and Neurodevelopmental Outcomes in Children From the Adolescent Brain Cognitive Development Study. Am J Psychiatry, 2020. 177(11): p. 1060-1072.
2. Honey, C.J., et al., Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A, 2009. 106(6): p. 2035-40.
3. Hutchison, R.M., et al., Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage, 2013. 80: p. 360-78.
4. Andersson, J.L., S. Skare, and J. Ashburner, How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 2003. 20(2): p. 870-88.
5. Jeurissen, B., et al., Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage, 2014. 103: p. 411-426.
6. Tournier, J.D., et al., MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage, 2019. 202: p. 116137.
7. Smith, R.E., et al., Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage, 2012. 62(3): p. 1924-38.
8. Smith, R.E., et al., SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage, 2015. 119: p. 338-51.
9. Power, J.D., et al., Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 2014. 84: p. 320-41.
10. Terasaki, L.S., J. Gomez, and J.M. Schwarz, An examination of sex differences in the effects of early-life opiate and alcohol exposure. Philos Trans R Soc Lond B Biol Sci, 2016. 371(1688): p. 20150123.
Figures