4022
Quantifying Infant Lean Body Mass using Free-Breathing 3D Stack-of-Radial MRI1David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, United States, 2Department of Bioengineering, University of California Los Angeles, Los Angeles, CA, United States, 3Department of Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States, 4Department of Pediatrics, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
Lean body mass (LBM) is an important measure of metabolic ability. Decreased LBM during infancy may be associated with impaired growth and increased risk for future metabolic disease. This study measured skeletal muscle area as a surrogate for LBM in infants using free-breathing MRI. Psoas and paraspinal muscle areas were measured in 14 infants at the L4-L5 intervertebral disc level. Cross-sectional area of the thigh muscles was measured at the right midthigh in 8 infants. Infants with higher birth weights and those born large for gestational age had larger muscle areas than infants born lighter and/or small for gestational age.
Introduction
Lean body mass (LBM) is an important measure of health and metabolic activity1. Decreased LBM during infancy may be associated with low birth weight, impaired growth, and increased risk for future metabolic disease2,3. Very few studies have examined LBM in infants. Most studies in adults measure fat-free mass using techniques that involve ionizing radiation, such as computed tomography (CT) and dual-energy X-ray absorptiometry (DXA), which are not appropriate in infants1,3. In contrast, MRI is safe for all age groups and can provide measurements of the individual components of body composition1. However, conventional Cartesian MRI requires the subject to remain still and hold their breath to avoid motion artifacts, which presents challenges for the pediatric population3.Recently, a new free-breathing 3D stack-of-radial MRI technology has been developed to measure body composition in children and infants without the need for breath-holding or sedation4,5. This study aims to measure LBM in infants using free-breathing 3D stack-of-radial MRI.
Methods
In this IRB-approved HIPAA-compliant study, 14 infants (Table 1) underwent free-breathing 3D stack-of-radial MRI at 3T (MAGNETOM Skyra or Prisma, Siemens) without sedation4. Representative imaging parameters included: Field-of-view: 150x150 mm to 250x250 mm, in-plane resolution: 1.30x1.30 mm to 1.56x1.56 mm, slice thickness: 2mm-3mm, number of slices: 40-72.Skeletal muscle mass is a large component of LBM and thus can be used as a proxy for LBM6,7. We adapted previous approaches6-9 to measure the bilateral psoas, bilateral paraspinal, and right thigh muscle cross-sectional areas. Cross-sectional psoas muscle and paraspinal muscle areas (cm2) were measured from a single axial MRI slice at the L4-L5 intervertebral disc level (Figure 1). Cross-sectional thigh muscle areas (cm2) were measured from a single oblique MRI slice at the midpoint of the right thigh (Figure 2). The midthigh point was measured between the top of the femoral head and bottom of the femoral condyles7. All measurements were performed by a researcher using Horos (The Horos Project) or OsiriX, and confirmed by a pediatric radiologist. Skeletal muscle measurements were compared with other markers of growth, including age and birth weight.
Results
Demographics and skeletal muscle measurements are summarized in Table 1. Psoas and paraspinal muscle areas were measured in 14 infants at the L4-L5 level. Muscles at the midthigh were measured in 8 infants. For each infant, psoas and paraspinal muscle area measurements tended to be similar to each other (Figure 3). Infants with heavier birth weights have larger measured psoas and paraspinal muscle areas on MRI (Figure 4). Infants who were born heavier also tended to have larger muscle area at midthigh. The infants who were born large for gestational age (LGA) had larger psoas and paraspinal muscle areas than the infants who were born small for gestational age (SGA), regardless of age of the infant at the time of the MRI scan (range between 2-7 months old).Discussion
There has been very little published data on infant body composition. Children, and particularly infants, present unique challenges in measuring LBM3. CT and DXA are not appropriate for research in infants due to ionizing radiation exposure1,3. The Pea Pod system uses air displacement plethysmography to assess body composition and is accessible and easy to use, but it does not match the level of detail found in MRI and cannot determine skeletal muscle mass10. In contrast, free-breathing MRI is a promising technology to measure infant body composition in a safe, practical way.The abdominal region is regularly used in adult body composition imaging studies7-9. Measurements from a single MRI slice at L4-L5 are appropriate surrogate measures for both whole-body skeletal muscle and adipose tissue8,9. Psoas, paraspinal, and midthigh muscle area measurements from one MRI slice have all been shown to correlate with measures of total LBM7. Both midthigh and L4-L5 are appropriate levels to measure LBM7.
We measured skeletal muscle area at representative slices on free-breathing MRI as one way to examine LBM in infants. Psoas, paraspinal, and thigh muscles were successfully manually segmented and measured. Our results showed that none of the muscle area measurements have a close relationship with the age of the infant at the time of the MRI scan. Additionally, LGA infants yielded larger muscle areas than SGA infants.
This study focused on LBM measurements, which can provide valuable information about body composition in the infants studied. Along with other measures of subcutaneous and visceral fat, having a way to measure LBM in infants can help characterize an infant’s risk for metabolic disease and obesity in the future2.
Conclusion
Using free-breathing 3D stack-of-radial MRI, it is feasible to measure skeletal muscle area at the L4-L5 level and midthigh as a proxy for LBM in infants. Additional research using this new tool is needed to characterize associations between infant LBM and other measures of body composition, maternal characteristics, and childhood development.Acknowledgements
This project was supported by the UCLA Radiological Sciences Exploratory Research Program, the NIDDK (R01DK124417), the NCATS (UL1TR001881), and the Short-Term Training Program at the David Geffen School of Medicine at UCLA. The authors thank Dr. Tess Armstrong for supporting this project.
References
- Prado, C. M., & Heymsfield, S. B. (2014). Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN. Journal of parenteral and enteral nutrition, 38(8), 940–953. https://doi.org/10.1177/0148607114550189
- Calkins, K., & Devaskar, S. U. (2011). Fetal origins of adult disease. Current problems in pediatric and adolescent health care, 41(6), 158–176. https://doi.org/10.1016/j.cppeds.2011.01.001
- Demerath, E.W., & Fields, D.A. (2014). Body composition assessment in the infant. American journal of human biology: the official journal of the Human Biology Council, 26(3), 291–304. https://doi.org/10.1002/ajhb.22500
- Armstrong, T., Ly, K. V., Ghahremani, S., Calkins, K. L., & Wu, H. H. (2019). Free-breathing 3-D quantification of infant body composition and hepatic fat using a stack-of-radial magnetic resonance imaging technique. Pediatric radiology, 49(7), 876–888. https://doi.org/10.1007/s00247-019-04384-7
- Ly, K. V., Armstrong, T., Yeh, J., Ghahremani, S., Kim, G. H., Wu, H. H., & Calkins, K. L. (2019). Free-breathing Magnetic Resonance Imaging Assessment of Body Composition in Healthy and Overweight Children: An Observational Study. Journal of pediatric gastroenterology and nutrition, 68(6), 782–787. https://doi.org/10.1097/MPG.0000000000002309
- Phillips, S.M., & Shulman, R.J. (2021). Measurement of body composition in children. Motil, K.J., & Hoppin, A.G.(Eds.), UpToDate. https://www.uptodate.com/contents/measurement-of-body-composition-in-children
- Morrell, G. R., Ikizler, T. A., Chen, X., Heilbrun, M. E., Wei, G., Boucher, R., & Beddhu, S. (2016). Psoas Muscle Cross-sectional Area as a Measure of Whole-body Lean Muscle Mass in Maintenance Hemodialysis Patients. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation, 26(4), 258–264. https://doi.org/10.1053/j.jrn.2016.02.002
- Lee, S. J., Janssen, I., Heymsfield, S. B., & Ross, R. (2004). Relation between whole-body and regional measures of human skeletal muscle. The American journal of clinical nutrition, 80(5), 1215–1221. https://doi.org/10.1093/ajcn/80.5.1215
- Shen, W., Punyanitya, M., Wang, Z., Gallagher, D., St-Onge, M. P., Albu, J., Heymsfield, S. B., & Heshka, S. (2004). Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. Journal of applied physiology (Bethesda, Md.: 1985), 97(6), 2333–2338. https://doi.org/10.1152/japplphysiol.00744.2004
- Carberry, A., Colditz, P. & Lingwood, B. (2010). Body Composition From Birth to 4.5 Months in Infants Born to Non-Obese Women. Pediatr Res 68, 84–88. https://doi.org/10.1203/PDR.0b013e3181df5421
Figures
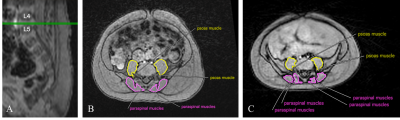
Figure 1. (A) Sagittal slice from free-breathing 3D MRI in an infant. Green lines depict the location of an axial MRI slice at the L4-L5 intervertebral disc level. (B) Axial MRI slice at the L4-L5 intervertebral disc level for an 11-week-old infant who was born at normal weight. Psoas and paraspinal muscles were contoured to calculate area. (C) Axial MRI slice at the L4-L5 intervertebral disc level for a 6-month-old infant who was born SGA. Psoas and paraspinal muscles were contoured to calculate area.
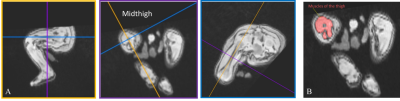
Figure 2. (A) Multiplanar oblique views of the thigh on free-breathing 3D MRI in a 2.5-month-old infant who was born SGA. Cross-sectional slices through the right thigh were aligned with the right femur shaft. (B) A cross-sectional slice through the right midthigh of the same infant. Muscles were contoured to calculate area.
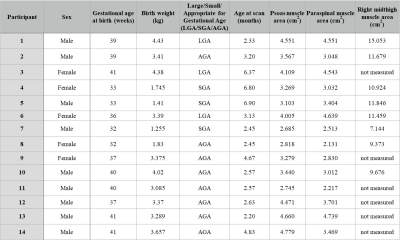
Figure 3. Measured area (cm2) from psoas, paraspinal and midthigh muscles for each infant. Psoas and paraspinal muscle areas were measured in all infants (n=14). A subgroup of 8 infants was used to measure midthigh muscle areas.
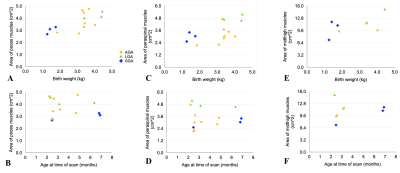
Figure 4. (A) Psoas muscle area at the L4-L5 level versus birth weight (n=14). (B) Psoas muscle area at L4-L5 versus age at time of scan (n=14). (C) Paraspinal muscle area at L4-L5 versus birth weight (n=14). (D) Paraspinal muscle area at L4-L5 versus age at scan (n=14). (E) Area of all muscles at the midthigh level versus birth weight (n=8). (F) Midthigh muscle area versus age at scan (n=8). Golden circles: infants born at a weight appropriate for gestational age (AGA). Green triangles: infants born large for gestational age (LGA). Blue diamonds: infants born small for gestational age (SGA).