4008
Histological validation of prostate tissue composition measurement using Hybrid Multidimensional MRI: Agreement with pathologists’ measures1Department of Radiology, University of Chicago, Chicago, IL, United States, 2Sanford J. Grossman Center of Excellence in Prostate Imaging and Image Guided Therapy, Chicago, IL, United States, 3Department of Pathology, University of Chicago, Chicago, IL, United States, 4Department of Pathology, Medical College of Wisconsin, Medical College of Wisconsin, WI, United States, 5JBS Consulting Services Inc., Carlsbad, CA, United States
Synopsis
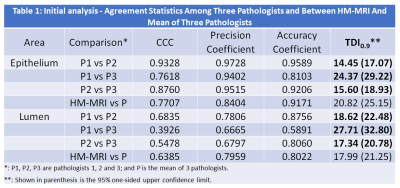
We validated prostate tissue composition measured using HM-MRI by comparing with reference standard results from pathologists’ interpretation of clinical histopathology slides following whole mount prostatectomy. We are 95% confident that 90% of absolute paired differences (TDI0.9) between HM-MRI and consensus results of pathologists were within 20.6% and 24.2% in measuring epithelium and lumen fractional volumes, respectively. These were less than our criterion of 30% and inter-pathologists’ agreement (22.3% for epithelium and 24.2% for lumen). Therefore, we accept the agreement performance of HM-MRI in measuring tissue composition measurement and consensus of pathologists is on par with the inter-raters (pathologists) agreement.
Introduction
Even though mpMRI is increasingly being used for prostate cancer (PCa) diagnosis, around 15-30% of clinically significant cancers are missed even by expert radiologists. Prostate tissue composition of gland components: stroma, epithelium, and lumen change with the presence (1) and Gleason grade of PCa (2). Therefore, the distinct MR properties of these tissue components (3) can be exploited to measure tissue composition changes non-invasively using MRI and be used as biomarker for non-invasive PCa detection.Recent studies have shown that prostate tissue composition can be measured non-invasively using Hybrid Multidimensional MRI (HM-MRI) and that this approach has the potential to improve prostate cancer diagnosis and determine its aggressiveness. However, our previous study on measurements of prostate tissue composition using HM-MRI (4) lacked validation with ground truth histopathology results. Therefore, the purpose of this study is to validate prostate tissue composition measured using HM-MRI by comparing with reference standard (ground truth) results from pathologists’ interpretation of clinical histopathology slides following whole mount prostatectomy.
Materials and Methods
In this prospective study, 36 participants (mean age = 60 years, mean PSA = 9.0 ng/ml) with biopsy-confirmed prostate cancer underwent MR imaging with a 3T Philips Achieva MR scanner prior to radical prostatectomy. The median time between MRI and prostatectomy was 20 days (range 1-34 days). Axial HM-MRI was acquired with all combinations of echo times of 57, 70, 150, 200 ms and b-values of 0, 150, 750, 1500 s/mm2 similar to previous works (4).Tissue composition were calculated by fitting the HM-MRI data to a three compartment signal model, with distinct, paired ADC and T2 values associated with each compartment, similar to the previous studies (5,6).
$$ \frac{S}{S_0} =\sum_{n=1}^{n=3} V_n \times exp (-ADC_n \times b - \frac{TE}{T2_n}) $$
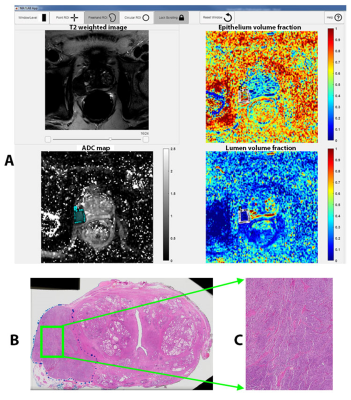
Three experienced genitourinary pathologists independently as well as in consensus reviewed each histology image and provide an estimate of percentage of epithelium and lumen for regions-of-interest corresponding to MRI. An initial review of 10 patients (38 samples) was done to obtain preliminary results needed for sample size (power analysis) and acceptance criterion determination. This was followed by independent analysis of the whole data set (n=165; 64 prostate cancers and 101 benign tissue) by each of the pathologists. Four weeks after the initial review, pathologists repeated the review of the initial 10 cases and recorded their results. This allowed us to calculate intra-observer variation for these tissue percentage estimates. Upon completion of the independent review by each pathologist, the three pathologists reviewed the ROIs in consensus for the estimates of epithelium and lumen and recorded the results. These results served as the ground truth measures for this cohort. Agreement statistics using total deviation index (TDI0.9) was performed for tissue composition measured using HM-MRI and reference standard results from pathologists’ consensus.
Results
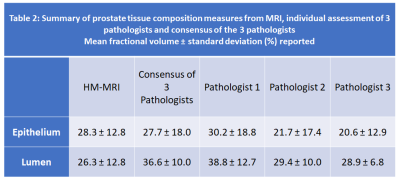
Based on the initial results (Table 1) showing typical variation among pathologists TDI0.9=25%, we determined we will declare acceptable agreement if the 95% one-sided upper confident limit of TDI0.9 is less than 30%.Representative example of the analysis is shown in Figure 1. Summary of prostate tissue composition measures from MRI, individual assessment of 3 pathologists and consensus of the 3 pathologists is shown in Table 2.
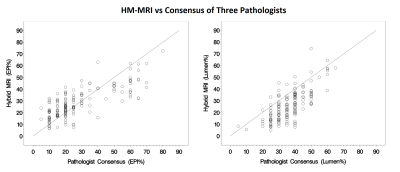
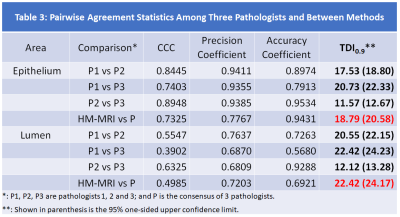
The results of tissue composition measurement from HM-MRI compared to ground truth results from the consensus of 3 pathologists, reveal that ninety percent of absolute paired differences (TDI0.9) were within 18.8% and 22.4% in measuring epithelium and lumen, respectively (Table 3). We are 95% confident that 90% of absolute paired differences were within 20.6% and 24.2% in measuring epithelium and lumen, respectively. These were less than our criterion of 30% and inter-pathologists’ agreement (22.3% for epithelium and 24.2% for lumen) and therefore we accept the agreement performance of HM-MRI. The agreement plots in measuring fractional volume of epithelium (EPI%) and lumen (Lumen%) using HM-MRI and consensus of 3 pathologists is shown in Figure 2.
Intra-reader agreement ranged from TDI0.9 = 6.6-14.3% (95% upper confidence limit = 8.0-17.3%).
The results revealed excellent area under the ROC curve for differentiating cancer from benign tissue based on epithelium (HM-MRI: 0.87, pathologists: 0.97) and lumen volume (HM-MRI: 0.85, pathologists: 0.77).
Discussion
The results of this study demonstrate that the tissue composition measured non-invasively using HM-MRI matches very closely with the reference standard results from the consensus of 3 expert pathologists. The tissue composition estimated non-invasively in this study using both HM-MRI and pathologists and the trend of cancers having increased epithelium and reduced lumen volume compared to benign tissue is seen here for both HM-MRI and pathologists’ measurement, which matches results in previous studies using morphometric analysis of H&E-stained prostate tissue and from other microstructure imaging methods such as luminal water imaging (5), VERDICT (6), etc.In addition, high area under the receiver operating characteristic curve using tissue composition measures from HM-MRI and pathologists suggests that HM-MRI can potentially be used for non-invasive prostate cancer diagnosis with prostate cancers characterized by increased epithelium and reduced luminal volume compared to benign tissue. Similar validation using different MR vendors and in a multicenter are also needed in the future.
Conclusion
The agreement in tissue composition measurement using hybrid-multidimensional MRI and consensus of pathologists is on par with the inter-raters (pathologists) agreement.Acknowledgements
This study was supported by NIH (R01 CA227036, 1R41CA244056-01A1, R01 CA17280, 1S10OD018448-01), Sanford J. Grossman Charitable Trust and University of Chicago Medicine Comprehensive Cancer Center (P30 CA014599-37).References
1. Langer DL, van der Kwast TH, Evans AJ, Plotkin A, Trachtenberg J, Wilson BC, Haider MA. Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology 2010;255(2):485-494.
2. Chatterjee A, Watson G, Myint E, Sved P, McEntee M, Bourne R. Changes in Epithelium, Stroma, and Lumen Space Correlate More Strongly with Gleason Pattern and Are Stronger Predictors of Prostate ADC Changes than Cellularity Metrics. Radiology 2015;277(3):751-762
3. Bourne RM, Kurniawan N, Cowin G, Stait-Gardner T, Sved P, Watson G, Price WS. Microscopic diffusivity compartmentation in formalin-fixed prostate tissue. Magn Reson Med 2012;68(2):614-620.
4. Chatterjee A, Bourne R, Wang S, Devaraj A, Gallan AJ, Antic T, Karczmar GS, Oto A. Diagnosis of Prostate Cancer with Noninvasive Estimation of Prostate Tissue Composition by Using Hybrid Multidimensional MR Imaging: A Feasibility Study. Radiology 2018;287(3):864-872.
5. Sabouri S, Fazli L, Chang SD, et al. MR measurement of luminal water in prostate gland: Quantitative correlation between MRI and histology. Journal of Magnetic Resonance Imaging 2017; 46:861-869
6. Panagiotaki E, Chan RW, Dikaios N, et al. Microstructural Characterization of Normal and Malignant Human Prostate Tissue With Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours Magnetic Resonance Imaging. Investigative radiology 2015; 50:218-227
Figures