4005
Navigator-based slice tracking for kidney pCASL using EPI acquisition1Department of Diagnostic and Interventional Radiology, Heidelberg University Hospital, Heidelberg, Germany, 2Department of Radiology, German Cancer Research Center, Heidelberg, Germany
Synopsis
Renal
perfusion is an important physiological parameter in health and disease (1).
To measure kidney perfusion
using arterial spin labelling (ASL) the respiratory motion is a major problem. In this study, respiratory
motion information is acquired from a projection signal and used to adjust the
position of the excited slice in real time. The feasibility of free-breathing
multi-slice kidney perfusion imaging using EPI
based pseudocontinuous ASL (pCASL)
with navigator-based slice tracking method is investigated.
PURPOSE
To apply the navigator-based slice tracking method to prospectively compensate the respiratory motion for kidney pseudo-continuous arterial spin labeling (pCASL) using EPI acquisition.METHODS
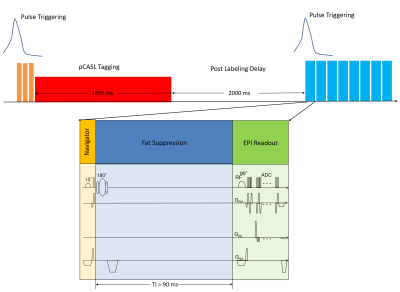
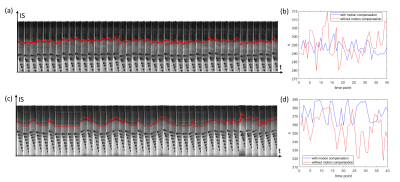
Measurements were performed using an 18-channel body and spine receive RF coil on a 1.5T Aera Siemens scanner (Siemens Healthineers AG, Erlangen, Germany). A single gradient-echo slice selection and projection readout at the location of the diaphragm along the inferior-superior (IS) direction is acquired as a navigator. Navigator acquisition and fat suppression were interleave inserted before each transverse imaging slice of the readouts of 2D gradient-echo EPI based pCASL sequence (Fig. 1), with 45 measurements including 4000 training navigators at the beginning of measurements obtained in 5 minutes. Sequence parameters were as follows: TE =12 ms, FOV=400×240 mm3, in-plane iPAT factor=3, matrix size=120×72×8, resolution=3.3×3.3×8 mm3, slice gap = 4 mm, labeling duration = 1650 ms, postlabeling delay (PLD) =2000 ms, TR = 6500 ms, TI for SPAIR (SPectral Attenuated Inversion Recovery) fat suppression=90 ms, FOVnav = 200 mm, resnav = 64, Flip anglenav = 15º, TRnav=4.22ms. The labeling pulse train and readout were triggered by the pulse signal and played out only during the systolic period. The renal blood flow was selectively tagged using a tagging plane placed above to the diaphragm, labeling the arterial blood in the descending aorta.Before motion analysis, the interleaved navigator signals during image acquisition were Fourier transformed and truncated to exclude RF saturation along IS direction from the EPI readout (Fig. 2). The position for this truncation was calculated based on peaks fitted from the averaged training navigator and the peaks from the first 32 interleaved navigators. The diaphragm position was derived by calculating the phase difference of the interleaved navigator signals at each acquisition after Fourier transform and truncation. The unwrapped data from different coils were then combined by using coil clustering (2) based on the first 32 interleaved navigators. The motion information was then directly sent back to the sequence and slice positioning was adjusted in real-time. This motion analysis and real-time feedback was performed on the scanner, implemented in ICE (Image calculation environment, Siemens Healthineers AG, Erlangen, Germany).
RESULTS
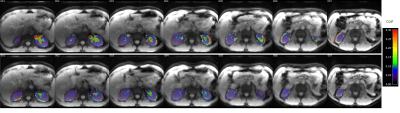
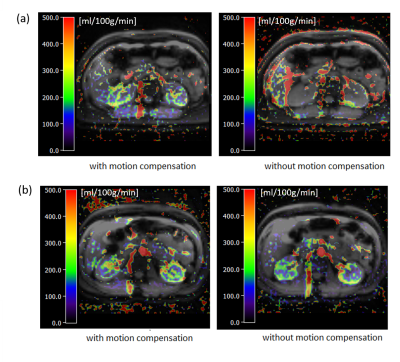
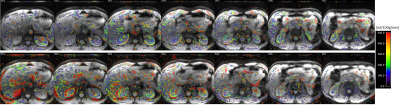
The respiratory motion from navigator signals could be precisely calculated (Fig. 2) and slice positioning was changed in real-time based on the motion information. Comparing to the case without, motion compensation reduced liver movement in a selected slice (Fig. 2). Fig. 3 shows spatial maps of the coefficient of variation (CoV) of control images from a represent volunteer in kidney. The value of CoV was calculated as the standard deviation of the constituent voxels divided by their mean. Motion greatly increased the CoV, but to a lesser extent when the motion correction was on. The calculated perfusion maps show significantly higher perfusion in the renal cortex from navigator-based motion compensated pCASL than from no motion compensated pCASL (Fig. 4, 5). More detailed structure in the perfusion maps could be observed in the motion compensated case (Fig. 4, 5).CONCLUSION
This study demonstrates the feasibility of navigator-based slice tracking technique in kidney pCASL using EPI readout. The sequence may improve the evaluation of the perfusion in kidney diseases.Acknowledgements
No acknowledgement found.References
1. Dalal R, Bruss ZS, Sehdev JS. Physiology, Renal Blood Flow and Filtration. StatPearls. Treasure Island (FL); 2021.
2. Zhang T, Cheng JY, Chen YX, Nishimura DG, Pauly JM, Vasanawala SS. Robust Self-Navigated Body MRI Using Dense Coil Arrays. Magn Reson Med 2016;76(1):197-205.
Figures