4004
Development of a MRI radiomic-based ML model to predict aggressiveness of prostate cancer1Radiology, Pontificia Universidad Catolica de Chile, Santiago, Chile, 2Physics, Pontificia Universidad Catolica de Chile, Santiago, Chile, 3Urology, Pontificia Universidad Catolica de Chile, Santiago, Chile
Synopsis
We developed a non-invasive tool to predict the GS classification of PCa based on mpMRI information using ML. This retrospective study included 86 male patients with positive PCa fusion (mpMRI-ultrasound) biopsy. A radiomic analysis was performed considering first order, textural, shape, and clinical information. The best model found included image (T2w - ADC) and clinical information. The mean AUC was 0.91 [0.75−0.99] (p <0.05), with a validation AUC of 0.91 for a classification of high-lower aggressiveness (GS≥7 vs GS=6). Combining MRI-based radiomic and clinical information can significantly improve the model performance to classify PCa aggressiveness.
Introduction:
The gold standard for the evaluation of prostate cancer (PCa) aggressiveness is the Gleason score (GS), which requires a histopathological analysis to discriminate between clinically significant (CS, GS≥ 7) and non-significant (non-CS, GS=6) cases (1). Patients with PCa are classified into a risk group based on their PSA level, pathological assessment/Gleason score, and clinical stage (2), which defines treatment. However, patient risk stratification constitutes a substantial challenge due to limitations of the current management algorithm, mainly the low specificity of serum PSA (3) and overdetection of low-grade clinically insignificant cancers by transrectal ultrasonography (TRUS)-guided biopsy with consequent overtreatment (4, 5). Noninvasive prediction of PCa aggressiveness is a substantial unmet need, which has led to the search for new methods that could lead to improved risk stratification and management, including the use of multiparametric magnetic resonance imaging (mpMRI) (6, 7). The aim of this study was to develop a non-invasive tool able to predict the GS classification of PCa, based on radiomic texture data extracted from mpMRI, by using machine learning (ML) tools. Additionally, the impact on the model performance of the feature selection method and the inclusion of clinical data and qualitative image information was assessed.Materials and Methods:
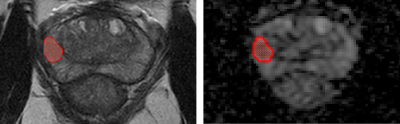
This retrospective cohort included 86 adult male patients with positive biopsy for PCa, made by fusion technique (mpMRI-ultrasound) at Hospital Clínico de la Pontificia Universidad Católica de Chile between 2017 and 2021, with lesions greater than 5 mm. 2D segmentations of the target prostate lesions were made by experienced radiologists in T2 weighted (T2w)/Apparent Diffusion Coefficient (ADC) map images at a 3T scanner (Figure 1). A radiomic analysis was performed considering first order, textural and shape features extracted from the segmented area, besides other clinical and imaging information (PSA values, prostate volume), including qualitative image information like PIRADS-v2.1(8). Splitting the dataset on train/test (80%) and validation sets (20%), univariate and multivariate models were built using manual and automatic feature selection algorithms. In order to evaluate the performance of the models, twofold cross-validation (CV) was employed with an 80%/20% split for the train/test groups, respectively. In particular, we used the Repeated Stratified KFold CV technique with 1000 repetitions, with the Area Under the Curve (AUC) as the evaluation metric. The manual selection method was based on individual feature performance and correlation, using parametric and non-parametric statistical hypothesis tests, Pearson correlation, and predictive power with bootstrap AUC analysis. A comparison between models was performed using Frequentist and Bayesian correlated t-tests.Results:
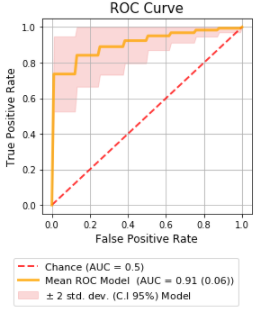
The best model found was multivariate, obtained using the automatic feature selection algorithm Recursive Feature Elimination (RFE), with Logistic Regression as an estimator with nine features, including image (T2w and ADC) and clinical information. Selected features include first order Maximum (ADC) and GLRLM, GLCM and NGTDM texture categories. The train/test mean AUC was 0.91 (0.06) [0.75−0.99] (p-value<0.05), with a validation AUC of 0.91 for a classification of high-lower aggressiveness (GS≥7 vs GS=6) (Figure 2). The Bayesian tests confirmed that our best model performed better than the best univariate model and multivariate models considering image features or clinical information only, with probability values of 0.94, 0.78 and 0.69 respectively, showing great superiority over the other two models.Discussion:
We performed this study to evaluate the potential of a radiomic MR-based ML algorithm to predict PCa aggressiveness. Our results showed that by incorporating image and clinical data into our model it was possible to discriminate with an adequate AUC between clinically significant versus non-clinically significant cancer, directly impacting patient management. There is vast evidence that supports the use of MRI-derived biomarkers in the risk stratification assessment of PCa patients (9, 10); however, there are several unmet needs for its widespread clinical application. Though previous studies show similar AUCs when evaluating texture-based data (11, 12), these studies lack an adequate ML methodology such as train/validation cohorts which was meticulously approached in this work. Successful integration of radiomic and clinical data into machine learning diagnosis tools and their introduction into clinical practice could make this process quantitative and less subjective to meet the increasing demands for MRI-directed diagnosis of PCa.Conclusion:
Our results show that a Radiomic ML approach is a valuable tool for the prediction of PCa aggressiveness (GS) by combining MRI-based radiomic and clinical information. Although further prospective studies in larger patient cohorts are needed, the results of our study suggest that Radiomic analysis has a potential role in the characterization of histological tumor grading of PCa, which could be valuable to direct patient management.Acknowledgements
This work was funded by ANID – Millennium Science Initiative Program – NCN17_129, and the National Fund for Scientific and Technological Development (FONDECYT) under Grant 1210648.References
(1) Epstein, J.I., et al., The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol, 2016. 40(2): p. 244-52.
(2) D'Amico, A.V., et al., Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol, 2003. 21(11): p. 2163-72.
(3) Ilic, D., et al., Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ, 2018. 362: p. k3519.
(4) Loeb, S., et al., Overdiagnosis and overtreatment of prostate cancer. Eur Urol, 2014. 65(6): p. 1046-55.
(5) Ahmed, H.U., et al., Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet, 2017. 389(10071): p. 815-822.
(6) Thompson, J., et al., The role of magnetic resonance imaging in the diagnosis and management of prostate cancer. BJU Int, 2013. 112 Suppl 2: p. 6-20.
(7) Yacoub, J.H., A. Oto, and F.H. Miller, MR imaging of the prostate. Radiol Clin North Am, 2014. 52(4): p. 811-37.
(8) Turkbey, B., et al., Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol, 2019. 76(3): p. 340-351.
(9) Siddiqui, M.M., et al., Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA, 2015. 313(4): p. 390-7.
(10) Kasivisvanathan, V., et al., MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med, 2018. 378(19): p. 1767-1777.
(11) Gillies, R.J., P.E. Kinahan, and H. Hricak, Radiomics: Images Are More than Pictures, They Are Data. Radiology, 2016. 278(2): p. 563-77.
(12) Nketiah, G.A., Elschot, M., Scheenen, T.W. et al. Utility of T2-weighted MRI texture analysis in assessment of peripheral zone prostate cancer aggressiveness: a single-arm, multicenter study. 2021, Sci Rep 11, 2085.