4002
Bilateral asymmetry of early parenchymal kinetics predicts response of breast cancer to neoadjuvant therapy1Radiology, The University of Chicago, Chicago, IL, United States, 2Medicine, The University of Chicago, Chicago, IL, United States, 3Research Computing Center, The University of Chicago, Chicago, IL, United States, 4Biomedical Engineering, The University of Texas at Austin,, Austin, TX, United States, 5Diagnostic Medicine, The University of Texas at Austin, Austin, TX, United States, 6Oncology, The University of Texas at Austin, Austin, TX, United States, 7Institute for Computational and Engineering Sciences, The University of Texas at Austin, Austin, TX, United States, 8Livestrong Cancer Institutes, The University of Texas at Austin, Austin, TX, United States, 9Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
We retrospectively reviewed data from 23 patients who received neoadjuvant therapy (NAT) and were scanned with a protocol that included ultrafast DCE-MRI (temporal resolution = 3-7 seconds) for the first minute after contrast injection prior to NAT. We measured parenchymal kinetics from ipsi- and contra-lateral normal parenchyma separately, so that new parameters related to bilateral parenchymal enhancement asymmetry could be calculated. The results showed that patients with similar pre-NAT parenchymal enhancement kinetics in ipsi- and contralateral normal parenchyma were more likely to achieve pCR post NAT ($$$p < 0.02$$$).
Introduction
Achieving a pathologic complete response (pCR) following neoadjuvant therapy (NAT) is strongly associated with improved overall and recurrence-free survival1. However, the overall pCR rate is only 21.1% and the response varies with breast cancer receptor subtype1. It is important to identify women who are not likely to achieve pCR prior to or early during NAT, so that alternative treatment regimens can be considered in the case of poor response2. Increasing evidence indicates that background parenchymal enhancement (BPE) could be a predictor of breast cancer response to NAT3. Yet, a consistent association between pre-NAT BPE and NAT response has not been convincingly demonstrated4-9. In this study, we introduce new ultrafast DCE-MRI parameters quantifying differences between ipsi- and contra-lateral parenchymal kinetics – and investigate their value for predicting the treatment outcome prior to administration of NAT.Method
Our IRB approved this retrospective study and waived written informed consent. Twenty-three female patients with histologically confirmed invasive breast cancer (grade II (n = 4) or III (n = 19)) who underwent ultrafast DCE-MRI (temporal resolution = 3 – 7 seconds) prior to NAT were enrolled. The median patient age = 55 (range 32 – 74). After completion of NAT, 12 patients achieved pCR and 11 patients were classified as residual cancer burden (RCB) I (n=4) or RCB II (n = 7). Patient age, tumor grade, and RCB scores were collected through review of medical records.Semi-automatic volumetric segmentation of whole breast and parenchyma was performed on the first pre-contrast image of ultrafast DCE-MRI using the ‘Level Tracking’ editing tool in 3D Slicer10. Vessel tracking/segmentation was performed on the maximum intensity projection along time direction of the subtraction images using a Hessian filtering method11. Tumors were manually segmented under the guidance of the radiologist. Vessel and tumor regions were extracted from parenchyma. A digital filter was applied to select top 20% most enhancing voxels in each of the ipsi-and contra-lateral normal parenchyma.
For each voxel within each top 20% region, we fit the percent signal relative enhancement $$$PSE(t)$$$ for the initial 60 seconds after contrast media administration to a truncated empirical mathematical model:
$$PSE (t)=(t\geq t_0) \cdot A\cdot \frac{(\alpha (t -t_0 )^2}{1+(\alpha (t -t_0 ))^2}, (1)$$
where $$$t$$$ is the bolus arrival time ($$$BAT$$$, s), $$$A$$$ is the upper limit of percent enhancement, and $$$\alpha$$$ is the uptake rate (s-1). An ROI within the aorta was selected to calculate the $$$BAT$$$ in the aorta using $$$Eq.(1)$$$. The $$$BAT$$$ in the parenchyma was then calculated relative to the $$$BAT$$$ in the aorta.
Given the parameters estimated from $$$Eq.(1)$$$, three secondary parameters were derived:
- $$$BPE30$$$ was calculated as the $$$PSE$$$ at 30 seconds post $$$BAT$$$ in aorta: $$$A\cdot \frac{(\alpha\cdot t)^2}{1+(\alpha\cdot t)^2}$$$, where $$$t= 30-t_0$$$, and $$$t_0$$$ is the $$$BAT$$$ in the voxel.
- Maximum
relative enhancement slope ($$$MaxSlope$$$, in units of s-1) was
the first derivative of $$$Eq.(1)$$$ when its second derivative is zero:$$$\frac{3\sqrt3}{8}A\cdot\alpha$$$.
- Initial area under the curve ($$$AUC30$$$) was calculated by integrating the signal enhancement curve given by $$$Eq.(1)$$$ for the first 30 seconds post $$$BAT$$$ in the voxel12: $$$A\cdot(t-\frac{\arctan(\alpha\cdot t)}{\alpha})$$$, where $$$t=30$$$ s.
Results
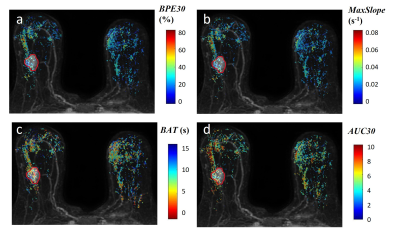
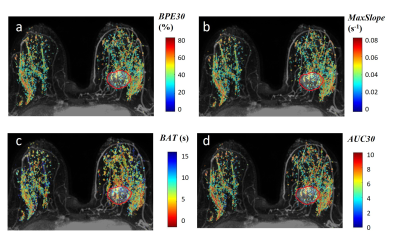
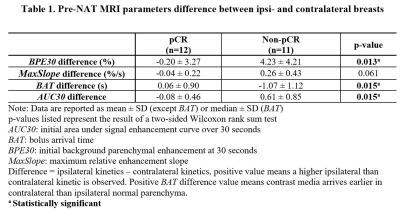
Kinetic parametric maps of two representative cases – a non-pCR patient and a pCR patient – are shown in Figures 1 and 2, respectively. Table 1 shows the bilateral difference in pre-NAT parenchymal kinetics, grouped by response to NAT. For the pCR group, the pre-NAT $$$BAT$$$ difference between two breasts was small ($$$0.06±0.9$$$ s). For the non-pCR group, the contrast bolus arrived (on median) $$$1.1±1.1$$$ seconds earlier in ipsilateral normal parenchyma than in contralateral normal parenchyma ($$$p=0.015$$$). Patients in the pCR group had similar ipsi- and contra-lateral pre-NAT $$$BPE30$$$ and $$$AUC30$$$, while patients in the non-pCR group had significantly higher pre-NAT mean $$$BPE30$$$ and $$$AUC30$$$ in ipsilateral than contralateral normal parenchyma ($$$p<0.02$$$). Moreover, the non-pCR group was trending toward a higher pre-NAT $$$MaxSlope$$$ in the ipsilateral normal parenchyma than in the contralateral parenchyma ($$$p=0.06$$$).Discussion
The results show that aggressive cancers significantly affect perfusion in the ipsilateral normal parenchyma, as reflected in rapid early uptake of contrast media measured by ultrafast DCE-MRI. Thus, ultrafast DCE-MRI may be able to identify aggressive cancers that require aggressive therapy. The comparison between the ipsi- and contra-lateral breast provides much more effective diagnostic and prognostic markers than features from unilateral breast, probably because the former corrects for physiologic variations. Bilateral asymmetry is consistent with recruitment of blood vessels to supply the cancer-containing breast, due to secretion of angiogenic factors by the cancer. This results in early contrast bolus arrival in tumor and in surrounding normal parenchymal tissue13 (Figure 1). More rapid enhancement in ipsilateral normal parenchyma indicates stronger angiogenic signaling, and a more aggressive cancer.Conlusion
High asymmetry between ipsi- and contra-lateral normal parenchyma could predict poor response to NAT.Acknowledgements
This study is supported in part by the National Cancer Institute of the National Institutes of Health through grants U01 CA142565, R01 CA172801, 1U24 CA226110, 1F32 CA265232, the Segal Family Foundation, the University of Chicago Cancer Center, and Cancer Prevention and Research Institute of Texas CPRIT RP160005. We also thank Dr. Olufunmilayo Olopade and the SPORE grant 5P20 CA233307-02. T.E.Y. is a CPRIT Scholar of Cancer Research.References
1. Spring LM, Fell G, Arfe A, et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin Cancer Res. 06 2020;26(12):2838-2848. doi:10.1158/1078-0432.CCR-19-3492
2. Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol. May 2012;30(15):1747-9. doi:10.1200/JCO.2011.41.3161
3. Liao GJ, Henze Bancroft LC, Strigel RM, et al. Background parenchymal enhancement on breast MRI: A comprehensive review. J Magn Reson Imaging. 01 2020;51(1):43-61. doi:10.1002/jmri.26762
4. Shin GW, Zhang Y, Kim MJ, et al. Role of dynamic contrast-enhanced MRI in evaluating the association between contralateral parenchymal enhancement and survival outcome in ER-positive, HER2-negative, node-negative invasive breast cancer. J Magn Reson Imaging. 12 2018;48(6):1678-1689. doi:10.1002/jmri.26176
5. Preibsch H, Wanner L, Bahrs SD, et al. Background parenchymal enhancement in breast MRI before and after neoadjuvant chemotherapy: correlation with tumour response. Eur Radiol. Jun 2016;26(6):1590-6. doi:10.1007/s00330-015-4011-x
6. Moliere S, Oddou I, Noblet V, Veillon F, Mathelin C. Quantitative background parenchymal enhancement to predict recurrence after neoadjuvant chemotherapy for breast cancer. Sci Rep. 12 2019;9(1):19185. doi:10.1038/s41598-019-55820-5
7. Choi JS, Ko ES, Ko EY, Han BK, Nam SJ. Background Parenchymal Enhancement on Preoperative Magnetic Resonance Imaging: Association With Recurrence-Free Survival in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy. Medicine (Baltimore). Mar 2016;95(9):e3000. doi:10.1097/MD.0000000000003000
8. Chen JH, Yu HJ, Hsu C, Mehta RS, Carpenter PM, Su MY. Background Parenchymal Enhancement of the Contralateral Normal Breast: Association with Tumor Response in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Transl Oncol. Jun 2015;8(3):204-9. doi:10.1016/j.tranon.2015.04.001
9. Xu C, Yu J, Wu F, et al. High-background parenchymal enhancement in the contralateral breast is an imaging biomarker for favorable prognosis in patients with triple-negative breast cancer treated with chemotherapy. Am J Transl Res. 2021;13(5):4422-4436.
10. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. Nov 2012;30(9):1323-41. doi:10.1016/j.mri.2012.05.001
11. Wu C, Pineda F, Hormuth DA, Karczmar GS, Yankeelov TE. Quantitative analysis of vascular properties derived from ultrafast DCE-MRI to discriminate malignant and benign breast tumors. Magn Reson Med. 03 2019;81(3):2147-2160. doi:10.1002/mrm.27529
12. Pineda FD, Medved M, Wang S, et al. Ultrafast Bilateral DCE-MRI of the Breast with Conventional Fourier Sampling: Preliminary Evaluation of Semi-quantitative Analysis. Acad Radiol. 09 2016;23(9):1137-44. doi:10.1016/j.acra.2016.04.008
13. Bogdanov A, Mazzanti ML. Molecular magnetic resonance contrast agents for the detection of cancer: past and present. Semin Oncol. Feb 2011;38(1):42-54. doi:10.1053/j.seminoncol.2010.11.002
Figures