4000
Assessment of Placental Perfusion in Normal and Hypertensive Pregnancies using pCASL at 3T: Preliminary Findings1Department of Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 2Department of Obstetrics and Gynecology, UT Southwestern Medical Center, Dallas, TX, United States, 3Department of Population and Data Sciences, UT Southwestern Medical Center, Dallas, TX, United States, 4Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 5Department of Bioengineering, University of Texas at Dallas, Richardson, TX, United States
Synopsis
Measuring placental perfusion can provide important information about its function. Arterial Spin Labeled (ASL) MRI is a non-contrast perfusion imaging method particularly suitable in pregnancy as it does not require an exogenous contrast. We performed a preliminary assessment of placental perfusion in pregnant subjects, both normal and with CHTN, at 16-20 week and 24-28 week gestational ages. Perfusion reduction was observed in all 5 normal subjects and in 75% (12 of 16) of the subjects with CHTN. This could provide important information about perfusion changes during pregnancy and can serve as a precursor for a larger-scale longitudinal study in CHTN.
Introduction
The human placenta delivers nutrients and oxygen playing a critical role for the developing fetus.[1][2] Measuring placental perfusion can provide important information about its function. Arterial Spin Labeled (ASL) MRI is a non-contrast perfusion imaging method particularly suitable in pregnancy as it does not require an exogenous contrast. Pseudo-continuous ASL (pCASL) is preferred for placental perfusion measurements as it measures the perfusion contribution from the maternal blood supply.[3][4] Placental perfusion may be affected by hypertension leading to increased resistance in the uterine artery and preventing the placenta from receiving enough blood flow, resulting in decreased placental perfusion and placental-mediated disease. In this study, we hypothesize that the baseline perfusion in pregnant hypertensive women would be lower and this decreased perfusion would persist.Methods
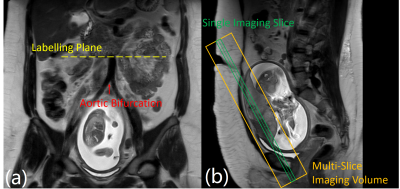
Inclusion criterion: singleton pregnant women who are healthy or with chronic hypertension (CHTN), body mass index (BMI) < 40kg/m2, gestational age < 20 weeks at recruitment, and no other complications. 5 normal and 16 women with CHTN were recruited. All underwent scans at 16-20 weeks (visit 1) and 24-28 weeks (visit 2) respectively.Imaging was performed on a 3T Philips Ingenia scanner. All were scanned under free-breathing in supine, feet-first position. pCASL labeling was used to selectively label the maternal abdominal aorta and was positioned perpendicularly above the level of the aortic bifurcation (Figure 1). Prior to ASL-MRI, T2-weighted images were acquired in coronal, axial, and sagittal planes to assist in the positioning of the labeling plane and imaging slices. Additional T2-weighted images matching the ASL imaging slices were also acquired in the coronal plane.
A multi-slice 2D pCASL and a multi-slice proton density weighted (M0) acquisition were performed for each scan using a 2D single-shot TSE (SShTSE). The imaging parameters of the 2D pCASL sequence were: TR/TE = 7500/38 ms, FOV = 275x335x54 mm3, acquired resolution = 3x3 mm2, slice thickness = 10 mm, number of signal averages (NSA) = 4, number of slices = 5, echo spacing = 5.5 ms, shot duration = 340 ms, echo train length (ETL) = 62, partial phase-encoding ratio = 0.55 along ky, receiver bandwidth = 307 Hz/pixel, label duration = 1.8 s, post-label delay = 1.8 s, 4 background suppression (BGS) pulses, and total scan time = 5 min. M0 images were acquired with the same parameters but with single average, and without labelling or BGS in 40 s. ASL images were reconstructed on the scanner using in-house-developed reconstruction which included complex k-space subtraction. The placental blood flow (PBF) values were quantified using the standard model for continuous ASL [5]. For each placenta, 2 polygon ROIs were drawn freehand on 2 selected slices of the T2-weighted images acquired at the same slices of the pCASL, and were used to calculate the mean blood flow value within the ROIs.
Results
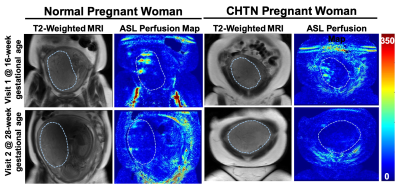
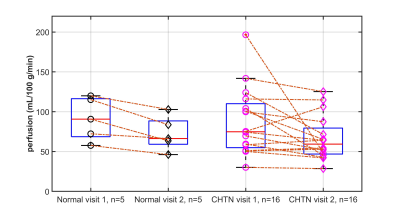
Placental perfusion observed in the spiral arteries of two representative subjects is shown in Figure 2. Overall, the placental perfusion decreased from visit 1 to visit 2 in both normal and CHTN women, although not statistically significant. The mean perfusion values at 16-20 weeks (visit 1) and 24-28 weeks (visit 2) gestation were 91.1 ± 26.8 and 72.5 ± 21.6 mL/100g/min in the normal cohort and 85.2 ± 42.5 and 67.4 ± 27.3 mL/100g/min in the CHTN cohort respectively. Perfusion decreased from visit 1 to visit 2 in all 5 normal women. Among the 16 women with CHTN, perfusion decreased in 12 subjects and substantially decreased in 1 woman (196 .6 and 48.5 mL/100g/min at visit 1 and visit 2 respectively), increased in 1 woman (75.3 and 106.2 mL/100g/min at visit 1 and visit 2 respectively), and remained unchanged (percentage changes <10%) in 3 women, showing an overall more variable perfusion changes in CHTN women (Figure 3).Discussion and Conclusion
Placental perfusion in women with CHTN, at 16-20 weeks (visit 1) and 24-28 weeks (visit 2), showed lower perfusion than normal, although not statistically significant. Perfusion reduction was observed in all the normal women and in 75% (12 of 16) of the women with CHTN. The variability in the CHTN group may be linked to the variability in maternal and fetal outcomes in this group and needs further evaluation. Future investigation will also evaluate regional variations. This study could provide important information about perfusion changes during pregnancy and can serve as a precursor for a larger-scale longitudinal study in CHTN.Acknowledgements
No acknowledgement found.References
[1] Wang Y, Zhao S. Vascular Biology of the Placenta. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. PMID: 21452443.
[2] Linduska N, Dekan S, Messerschmidt A, Kasprian G, Brugger PC, Chalubinski K, Weber M, Prayer D. Placental pathologies in fetal MRI with pathohistological correlation. Placenta. 2009 Jun;30(6):555-9. doi: 10.1016/j.placenta.2009.03.010. Epub 2009 Apr 25. PMID: 19394080.
[3] Do Q, Herrera C, Lewis M, Xi Y, Spong CY, Twicker DM, Madhuranthakam AJ et al. Quantitative Perfusion Measurements of the Human Placenta with FAIR and pCASL Arterial Spin Labeling at 3T: Initial Feasibility. ISMRM 2020: 0577.
[4] Shao X, Liu D, Martin T, Chanlaw T, Devaskar SU, Janzen C, Murphy AM, Margolis D, Sung K, Wang DJJ. Measuring human placental blood flow with multidelay 3D GRASE pseudocontinuous arterial spin labeling at 3T. J Magn Reson Imaging. 2018 Jun;47(6):1667-1676. doi: 10.1002/jmri.25893. Epub 2017 Nov 14. PMID: 29135072; PMCID: PMC5951737.
[5] Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015 Jan;73(1):102-16. doi: 10.1002/mrm.25197. Epub 2014 Apr 8. PMID: 24715426; PMCID: PMC4190138.
Figures