3988
Differences in functional connectivity in older adults with mild cognitive impairment and subjective cognitive decline
Arunan Srikanthanathan1, Susan Vandermorris1, Nicolaas Paul Verhoeff1, Jean Chen1, Nathan Herrmann2, and Linda Mah1
1Rotman Research Institute , Baycrest Health Sciences, North York, ON, Canada, 2Sunnybrook Research Institute, Toronto, ON, Canada
1Rotman Research Institute , Baycrest Health Sciences, North York, ON, Canada, 2Sunnybrook Research Institute, Toronto, ON, Canada
Synopsis
Subjective cognitive decline (SCD) is a preclinical stage of Alzheimer’s disease (AD) that may precede mild cognitive impairment (MCI), a prodrome of AD. We used resting-state functional magnetic resonance imaging to examine the pattern of functional connectivity in anterior brain regions that support memory processes in SCD and MCI. We found reduction of posterior cingulate cortex functional connectivity with medial temporal lobe structures in MCI and SCD, and associated increases in functional connectivity in the anterior brain regions, although direct group comparisons of the latter did not yield statistically significant differences.
Introduction
Subjective cognitive decline (SCD) is a preclinical stage of Alzheimer’s disease (AD) describing individuals with cognitive concerns despite normal performance on neuropsychological tests or other formal testing1. Neuroimaging studies show that functional connectivity of neural networks is altered in AD prior to amyloid deposition, beginning in the posterior default mode network (pDMN)2. These findings have led to the cascading failure hypothesis of AD which suggests that failure begins in the pDMN which shifts the processing burden to other connectivity hubs found in the anterior-dorsal regions of the brain2. Consistent with this model, hypoconnectivity in the pDMN is evident in individuals with mild cognitive impairment (MCI)3, a prodromal stage in AD. We recently reported reduction of functional connectivity between the posterior cingulate cortex and left posterior parahippocampal gyrus in SCD, compared to older adults who were cognitively unimpaired (CU)4. However, functional connectivity within the anterior brain regions was not assessed. In the current study, we examined the pattern of connectivity changes in anterior brain regions in SCD and MCI. We hypothesized that SCD would show decreased functional connectivity between posterior cingulate cortex and medial temporal lobe structures compared to CU, but greater functional connectivity between these regions compared to MCI. We also predicted that SCD would show increased functional connectivity between the anterior cingulate cortex and medial prefrontal cortex regions compared to CU, but decreased functional connectivity between these regions compared to MCI.Methods
The sample included 72 participants with MCI, SCD or CU. CU (n=26) reported no memory concerns and performed within normal limits on neuropsychological (NP) assessments. SCD (n=29) was established by affirmative responses to “Do you feel like your memory is becoming worse?” “If so, are you worried?” and normal NP test performance1. MCI (n=17) was based on the presence of subjective cognitive concerns and impairment on ≥ 2 NP measures within a cognitive domain, and functional independence5.A 6-minute resting-state functional magnetic resonance imaging scan in an awake state with their eyes closed was acquired. The CONN Toolbox6 was utilized to compute connectivity matrices between each ROI (region-of-interest) pair to determine population level-estimates for SCD and CU groups. Within-group seed analysis with posterior cingulate cortex seed and medial temporal lobe structures (left and right posterior parahippocampal gyrus and hippocampus) as target ROIs was performed. Additional seed analysis with anterior cingulate cortex seed and medial prefrontal cortex as the target ROI was also performed within groups. A planned t-test was performed to confirm the previous findings of reduced posterior cingulate cortex-left posterior parahippocampal gyrus in SCD compared to CU4. ANOVA was performed using Statistical Program for Social Sciences for Windows (Version 26) to compare amongst groups CONN-generated correlation coefficients which served as measures of posterior cingulate cortex-medial temporal lobe and anterior cingulate cortex-medial prefrontal cortex functional connectivity.
Results
Based on the within-group seed analysis conducted using the CONN toolbox, the posterior cingulate cortex was found to have significant functional connectivity with the left and right posterior parahippocampal gyrus (T(25)= 5.7,p-FDR=0.0003;T(25)=3.02,p-FDR=0.0242 respectively), and left and right hippocampus (T(25)=4.36,p-FDR=0.0016;T(25)=4.07,p-FDR=0.0029 respectively) in the CU group, but not in either SCD or MCI. In contrast, significant connectivity between anterior cingulate cortex and medial prefrontal cortex was found in SCD and MCI groups (T(28)=2.39,p-FDR=0.0411;T(16)=2.89,p-FDR=0.0241 respectively) but not in the CU group.ANOVA showed that posterior cingulate cortex-right hippocampus functional connectivity was significantly different amongst groups (F(2,69)=3.408,p=0.039). Post-hoc analyses using Tukey’s post hoc criterion for significance indicated that functional connectivity between the posterior cingulate cortex and right hippocampus differed between CU and MCI (p=0.042). However, no differences were found between SCD and CU or MCI. There were no group effects on functional connectivity between the posterior cingulate cortex and left hippocampus (F(2,69)=1.090,p=0.342). ANOVA also showed no significant group effects on functional connectivity between anterior cingulate cortex and medial prefrontal cortex (F(2,69)=0.521,p=0.596) or posterior cingulate cortex and bilateral posterior parahippocampal gyri (F(2,69)=2.805,p=0.067 (left);F(2,69)=0.228,p=0.796 (right)).
Discussion
Our findings are broadly consistent with the cascading failure hypothesis2 suggesting reduction of posterior cingulate cortex functional connectivity with medial temporal lobe structures in MCI and SCD, and compensatory increases in functional connectivity in the anterior DMN, although direct group comparisons of the latter did not yield statistically significant differences. Limitations of this study includes relatively small sample sizes (i.e., MCI group) which may explain the latter and other negative findings, and the lack of AD biomarkers and longitudinal follow-up to establish SCD as a preclinical AD group. Thus, our findings require replication with longitudinal studies including larger sample sizes.Conclusion
The current findings of reduced connectivity between the pDMN and medial temporal lobe structures in older adults at risk for AD suggest the possibility of assessment of functional connectivity as a means to predict AD risk in SCD, as well as targeting of pDMN functional connectivity as a potential intervention for older adults at risk for developing AD.Acknowledgements
This work was funded by the Alzheimer Society of Canada, Alzheimer Society Research Program 16-03, Centre for Aging and Brain Health Innovation and Researcher-Clinician Partnership Program 2.References
- Jessen, F., Amariglio, R. E., Van Boxtel, M., Breteler, M., Ceccaldi, M., Chételat, G., ... & Subjective Cognitive Decline Initiative (SCD‐I) Working Group. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimer's & dementia, 10(6), 844-852.
- Jones, D. T., Knopman, D. S., Gunter, J. L., Graff-Radford, J., Vemuri, P., Boeve, B. F., ... & Jack Jr, C. R. (2016). Cascading network failure across the Alzheimer’s disease spectrum. Brain, 139(2), 547-562.
- Eyler, L. T., Elman, J. A., Hatton, S. N., Gough, S., Mischel, A. K., Hagler, D. J., ... & Kremen, W. S. (2019). Resting state abnormalities of the default mode network in mild cognitive impairment: a systematic review and meta-analysis. Journal of Alzheimer's Disease, 70(1), 107-120.
- Sharma, N., Murari, G., Vandermorris, S., Verhoeff, N. P. L., Herrmann, N., Jean Chen, J., & Mah, L. (2021). Functional Connectivity Between the Posterior Default Mode Network and Parahippocampal Gyrus Is Disrupted in Older Adults with Subjective Cognitive Decline and Correlates with Subjective Memory Ability. Journal of Alzheimer's Disease, (In Press), 1-11.
- Roberts, R., & Knopman, D. S. (2013). Classification and epidemiology of MCI. Clinics in geriatric medicine, 29(4), 753-772.
- Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity, 2(3), 125-141.
Figures
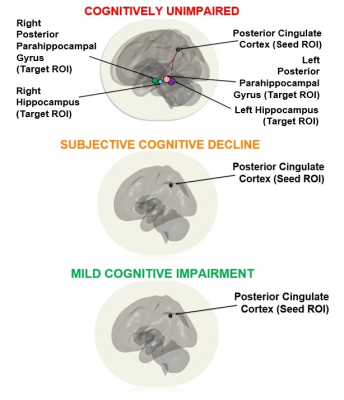
Figure 1. Within-group analysis using posterior cingulate cortex seed demonstrated significant functional connectivity between the posterior cingulate cortex seed and all 4 target ROIs - bilateral hippocampi and bilateral posterior parahippocampal gyri in the cognitively unimpaired group. These connections were not significant in both SCD and MCI groups.
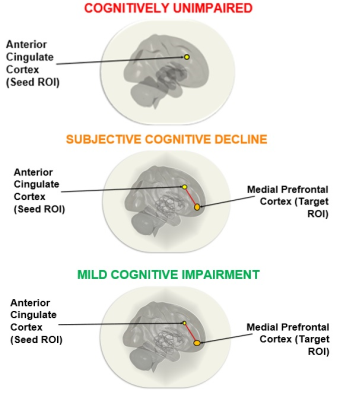
Figure 2. Within-group analysis
using the anterior cingulate cortex seed demonstrated significant functional
connectivity between the anterior cingulate cortex seed and the medial
prefrontal cortex in SCD and MCI groups. These connections were not significant
in the CU group.
DOI: https://doi.org/10.58530/2022/3988