3985
Glymphatic System Evaluation in Obstructive Sleep Apnea Adults using Diffusion Tensor Imaging1Anesthesiology, University of California Los Angeles, Los Angeles, CA, United States, 2Medicine, University of California Los Angeles, Los Angeles, CA, United States, 3Radiological Science, University of California Los Angeles, Los Angeles, CA, United States, 4Bioengineering, University of California Los Angeles, Los Angeles, CA, United States, 5Brain Research Institute, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
OSA is characterized by recurrent episodes of complete or partial collapse of the upper airway during sleep, leading to sleep fragmentation. The altered sleep architecture can potentially hinder the extracellular waste removal system known, the glymphatic system, which is known for clearing interstitial solutes, including beta amyloids. In this study, we evaluated the glymphatic system using non-invasive MRI based diffusion tensor imaging data analyses along the perivascular space (DTI-ALPS) in OSA patients and healthy controls, and show impaired glymphatic system in the condition.
Purpose
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of complete or partial collapse of the upper airway, with continuous diaphragmatic effort to breath during sleep, which results in either complete or partial pauses in breathing, creating cycles of O2 desaturation and re-oxygenation, sympathetic over-activity, and intra-thoracic pressure changes, leading to sleep fragmentation. OSA subjects show autonomic, mood, and cognitive deficits in several domains, and the condition is linked with increased risks for dementia, including Alzheimer’s disease (AD).1-3 The altered sleep architecture in OSA can potentially hinder glymphatic system, which is a sleep assisted highly polarized cerebrospinal fluid (CSF) and interstitial fluid transport system. Glymphatic system is a crucial brain clearance system of beta amyloid, a metabolic neuronal activity waste involved in AD pathogenesis, and is dependent on fluid transport between perivascular and interstitial spaces to wash out waste from tissue.4 Several studies show enhanced glymphatic system activities during sleep, and sleep issues are linked with reduced beta amyloid clearance potentially mediated through impaired glymphatic system.4,5 Although OSA subjects have increased risks for AD, it is unclear whether glymphatic system is impaired in the condition. Our purpose was to investigate the glymphatic system using non-invasive diffusion tensor imaging in OSA patients compared to healthy controls and to examine relationships between OSA disease severity, sleep symptoms, and glymphatic system indices.Materials and methods
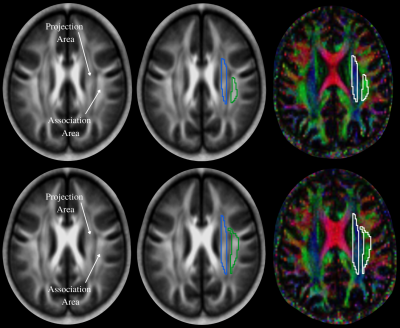
We examined 59 OSA (age, 49.9±10.0 years; body-mass-index (BMI), 31.8±5.5 kg/m2; 35 male; AHI, 35.4±21.0; SaO2 nadir, 78.5±9.5%; ΔSaO2, 16.1±9.1%), and 62 healthy controls (age, 50.1±10.4 years; BMI, 26.2±3.5 kg/m2; 34 male), using a 3.0-Tesla MRI (Siemens, Magnetom, Prisma Fit). Diffusion tensor imaging data were acquired using a single-shot echo-planar imaging with twice-refocused spin-echo pulse sequence (TR=10,000 ms; TE=87 ms; flip-angle=90° band-width=1346 Hz/pixel; matrix-size=128×128; FOV=230×230 mm; slice-thickness = 2.0 mm, b= 0 and 800 s/mm2, diffusion directions=30). Two self-administered questionnaires, Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS) were used to investigate sleep quality and daytime sleepiness in OSA and control subjects. We used diffusion (b=800 s/mm2)-weighted images, and non-diffusion (b = 0 s/mm2) images to compute diffusion tensor matrices using DTI-Studio software.6 Diffusivity maps in the direction of the x-axis (Dxx), y-axis (Dyy), and z-axis (Dzz) were calculated, in addition to Dxy, Dyz, and Dxz. The diffusivity maps (Dxx, Dyy, Dzz, Dxy, Dyz, and Dxz) were normalized to Montreal Neurological Institute (MNI) space. Non-diffusion weighted (b0) images were normalized to MNI space using a unified segmentation approach, and the resulting normalization parameters were applied to the diffusivity maps. Two sets of regions of interest (ROI) were placed at the level of the lateral ventricle body in the area of the projection and association fibers (Fig. 1) on the normalized diffusivity maps. The ROIs provided the value of Dxx, Dyy, Dzz, Dxy, Dyz, and Dxz at projection and association fibers from each subjects. Since all subjects were right-handed, we obtained measurements only in the left hemisphere as superior longitudinal fascicles and corona radiate are thicker on the dominant side. Considering Dxx and Dyy in the area of projection fibers as Dxxpro and Dyypro, respectively, and Dxx and Dzz at association fibers as Dxxasc and Dzzasc, respectively, ALPS index,7 which refers to DTI analysis along the perivascular space was defined as: ALPS index = [(Dxxpro+Dxxasc)/2]/[(Dyypro+Dzzasc)/2], where (Dxxpro+Dxxasc)/2 is expressed as Dxmean and (Dyypro+Dzzasc)/2 as Dyzmean. Demographic and clinical data were assessed between groups using independent samples t-tests for normally distributed data, and nonparametric Mann-Whitney U tests, for non-normally distributed data, and Chi-square test for categorical characteristics (SPSS, v27.0). Kolmogorov-Smirnov test with a p-value <0.05 was used to determine normality of the distribution. Spearman's correlations were used to determine associations between OSA severity, oxygen saturation variables, sleep symptoms, and diffusion indices in projection and association fiber areas within OSA subjects. A value of P<0.05 was chosen to establish statistical significance.Results
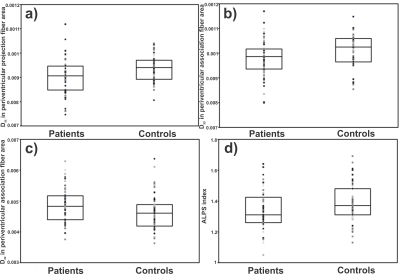
No significant differences in age (p=0.91) and sex (p=0.62) appeared between groups. However, the BMI (p<0.001) was significantly different between OSA and controls. The PSQI and ESS scores were significantly higher in OSA over controls (PSQI, p<0.001; ESS, p<0.001). Dzz values derived from projection fiber area are significantly reduced in OSA compared to control subjects (p=0.005) (Fig.2). Diffusion changes in association fiber area shows significant reduction in Dyy (p=0.02) and Dzz matrices (p=0.016) (Fig.2). ALPS (p=0.02) and Dyzmean (p=0.03) values were significantly different between OSA and control subjects. In periventricular projection fiber areas, Dxy values were correlated with ESS scores (r=0.29, p=0.03), Dxx values with SaO2 (r=-0.34, p=0.009) and ΔSaO2 (r=0.35, p=0.006), and at association fiber areas, relationships were observed between Dzz values and AHI (r=0.29, p=0.03) scores.Discussion
OSA subjects showed impaired glymphatic system based on non-invasive MRI based diffusion tensor imaging. In addition, significant differences in diffusion indices along the projection and association fibers emerged between groups. These indices correlated with OSA severity, O2 desaturation, and sleep symptoms. The abnormalities in projection and association fibers and ALPS index along with their associations with sleep parameters might explain the effects of OSA on brain glymphatic system dysfunction.Conclusions
OSA patients show abnormal glymphatic system function. The DTI-APLS method can be used to assess the activity of glymphatic system in patient with OSA.Acknowledgements
This work was supported by National Institutes of Health R01 HL-113251 and R01 NR-015038.References
1. Andrade A, Bubu OM, Varga AW, et al. The relationship between obstructive sleep apnea and Alzheimer’s disease. J Alzheimers Dis. 2018; 64: S255–S270.
2. Liguori C, Romigi A, Nuccetelli M, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer's disease biomarkers changes. Sleep. 40:zsx011.
3. Elias A, Cummins T, Tyrrell R, et al. Risk of Alzheimer's disease in obstructive sleep apnea syndrome: amyloid-β and tau imaging. J Alzheimers Dis. 2018; 66, 733–741.
4. Jessen NA, Munk ASF, Lundgaard I, et al. The glymphatic system – a beginner's guide. Neurochem Res. 2015; 40: 2583–2599.
5. Reddy OC, Werf YD. The sleeping brain: harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020; 10: 868.
6. Jiang H, Van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006; 81, 106–116.
7. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017; 35:172–178.
Figures