3972
Investigation of Blood-Brain Barrier Disruption in Schizophrenia using Magnetization Transfer-ASL at 7T.1Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 2Department of Psychiatry, University of Alabama at Birmingham, Birmingham, AL, United States
Synopsis
Schizophrenia (SCZ) is a central nervous system (CNS) disease which is characterized by thinking disorder, cognitive impairments and other clinical manifestations that alter daily functioning. SCZ currently does not have satisfactory treatment options to improve cognitive symptoms. Deeper understanding of the underlying mechanism is required for effective treatment of SCZ. Blood-brain barrier (BBB) is the immunological junction between brain and vascular circulation, and its disruption is associated with a number of CNS diseases. This study aims to investigate if the BBB is compromised in SCZ.
Introduction
The blood-brain barrier (BBB) regulates the nutrient transport between intra and extravascular space, and protects the central nervous system (CNS) from potentially harmful molecules. Integrity of the BBB plays a vital role in proper functioning of the brain, as BBB is the immunological interface between the brain and its surroundings1. Neuroimmune imbalance is implied in neuropsychiatric diseases such as schizophrenia (SCZ)2,3. SCZ is a pathoetiologically heterogeneous disorder that involves interrelated mechanisms such as oxidative stress and neuroinflamation. The BBB contributes to the CNS immune privilege as well as the oxygen regulation, and the barrier function has been implicated to be altered in SCZ4,5. We have previously demonstrated a non-invasive MRI technique to assess the BBB permeability using arterial spin labeling (ASL) combined with magnetization transfer (MT)6. Brain and vascular water experience different MT effect. Saturation of macromolecules during ASL experiment allows us to differentiate the vascular water from the tissue water and hence allows us to estimate the fraction of arterial water (E) that exchanges with the tissue. This technique can be used to measure perfusion and BBB permeability surface area product (PS). The aim of the present study was to determine whether altered perfusion and BBB permeability can be identified in SCZ patients. The motivation is that identifying different types pathological changes in brain may improve our understanding of the disease mechanisms.Methods
All the experiments were performed using Siemens 7T Magnetom with 32 channel head coil. Pulse sequence including QUIPSS II FAIR ASL technique with additional MT pulses were used to calculate perfusion, water extraction fraction, permeability surface area product and magnetization transfer ratio (MTR) as described before6.Control and SCZ patients (n=8 and 11 slices in each group) participated the study. 8 ms hyperbolic secant adiabatic inversion pulse was used to achieve slice selective and non-selective inversions. Other imaging parameters were: FOV= 256 mm, TE=1.39 s, TR=2 s, slice thickness=8mm, TI1=0.8 s, TI2=1.8 s, flip angle=100. 2.56 ms double saturation was used at TI1 to saturate the blood. MT pulse with duration=16.64 ms and offset frequency=500 Hz was used to saturate the macromolecules. The images were acquired in the order: control (MT on)/ tag (MT on)/ control (MT off)/ tag (MT off). A total pf 120 images (30 for each control (MT on), tag (MT on), control (MT off) and tag (MT off)) were acquired. A reference image with TR=2 s was acquired to normalize the perfusion images.
Data was transferred off line for processing. Perfusion and BBB maps were generated using custom software written in MATLAB as described before6. Gray and white matter were segmented using SPM and mean ± SD values were determined.
Results
Average perfusion (f) in SCZ patients was lower by 10% and 11% in gray matter and white matter respectively compared to the control group. PS was higher in the SCZ group by approximately 13% and 7% in gray and white matter respectively. Perfusion and BBB permeability (E and PS) results are summarized in table 1. Representative reference image, perfusion map, extraction fraction map and PS map from control subject and SCZ patient are shown in Fig. 1 and 2 respectively.Duscussion
Our results show that the BBB is disrupted in SCZ patients. Perfusion was found to be lower in SCZ patients compared to the controls, which supports the previous reports7,8. Higher permeability of the BBB is also consistent with the previous investigations9,10. Although average GM and WM BBB permeability were evaluated in this study, regional comparison might provide further insight about the underlying mechanism of SCZ disease.Acknowledgements
No acknowledgement found.References
1. Cheslow L, Alvarez JI. Glial-endothelial crosstalk regulates blood-brain barrier function. Curr Opin Pharmacol 2016;26:39-46.
2. Aleksovska K, Leoncini E, Bonassi S, Cesario A, Boccia S, Frustaci A. Systematic review and meta-analysis of circulating S100B blood levels in schizophrenia. PLoS One 2014;9(9):e106342.
3. Chen S, Tian L, Chen N, Xiu M, Wang Z, Yang G, Wang C, Yang F, Tan Y. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res 2017;247:6-11.
4. Cai HQ, Catts VS, Webster MJ, Galletly C, Liu D, O'Donnell M, Weickert TW, Weickert CS. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry 2020;25(4):761-775.
5. Severance EG, Gressitt KL, Alaedini A, Rohleder C, Enning F, Bumb JM, Muller JK, Schwarz E, Yolken RH, Leweke FM. IgG dynamics of dietary antigens point to cerebrospinal fluid barrier or flow dysfunction in first-episode schizophrenia. Brain Behav Immun 2015;44:148-158.
6. Mahmud SZ, Denney TS, Bashir A. Measurement of Blood-Brain Barrier Permeability in Human Brain using Magnetization Transfer Effect at 7T. In Proceedings of 28th Annual Meeting of ISMRM, 2020. p 1749.
7. Mathew RJ, Duncan GC, Weinman ML, Barr DL. Regional cerebral blood flow in schizophrenia. Arch Gen Psychiatry 1982;39(10):1121-1124.
8. Pinkham A, Loughead J, Ruparel K, Wu WC, Overton E, Gur R, Gur R. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res 2011;194(1):64-72.
9. Crockett AM, Ryan SK, Vasquez AH, Canning C, Kanyuch N, Kebir H, Ceja G, Gesualdi J, Zackai E, McDonald-McGinn D, Viaene A, Kapoor R, Benallegue N, Gur R, Anderson SA, Alvarez JI. Disruption of the blood-brain barrier in 22q11.2 deletion syndrome. Brain 2021;144(5):1351-1360.
10. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry 2020;10(1):373.
Figures
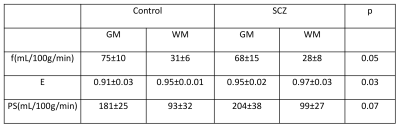
Table 1: Perfusion (f), water extraction fraction (E) and permeability surface area product (PS) values for control and SCZ patients.
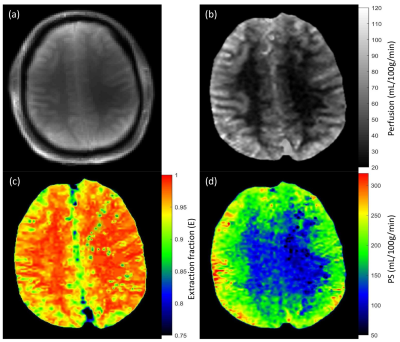
Figure 1: Reference proton density image (a) and corresponding perfusion (b), extraction fraction (c) and permeability surface area product (d) maps from healthy control.
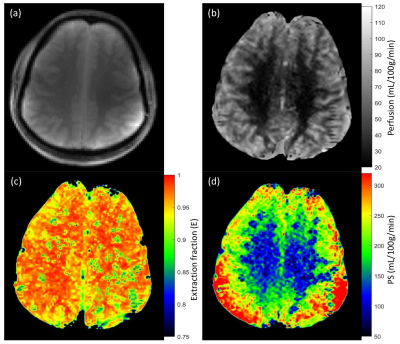
Figure 2: Reference proton density image (a) and corresponding perfusion (b), extraction fraction (c) and permeability surface area product (d) maps from Schizophrenia patient.